- Corresponding Author:
- Irene Furtado
Department of Microbiology, Goa University, Taleigao Plateau, Goa-403 206, India
E-mail: ijfurtado@unigoa.ac.in
| Date of Submission | 09 May 2012 |
| Date of Revision | 30 August 2012 |
| Date of Acceptance | 30 August 2012 |
| Indian J Pharm Sci, 2012, 74 (4): 331-338 |
Abstract
Marine ecosystem and its organisms, particularly the invertebrates are recent targets of bioprospecting and mining for a large group of structurally unique natural products encompassing a wide variety of chemical classes such as terpenes, polyketides, acetogenins, peptides and alkaloids of varying structures, having pronounced pharmacological activities. In view of the limited reports on the antibacterials produced by bacteria, isolated from marine sponges, corals and bivalves of Indian origin, the present study is aimed at investigating the antagonistic activities of 100 heterotrophic, halophilic bacterial bionts isolated from 9 sponges, 5 corals and one bivalve. Culture broths of 46 of these bionts were active against human pathogenic bacteria namely Staphylococcus citreus, Proteus vulgaris, Serratio marcesans, Salmonella typhi, Aerobacter aerogenes and Escherichia coli. Further, the ethyl acetate extracts of cell free supernatant confirmed the presence of extracellular bioactive factor, by agar cup diffusion method. Interestingly, highest number of bionts having activity was isolated from corals followed by sponges and bivalve. The study clearly demonstrates that bacterial bionts of marine invertebrates are a rich source of bioactive secondary metabolites against human bacterial pathogens.
Keywords
Antibacterial, halophilic-bacterial-bionts, human pathogens
Soil has been widely explored as the source of microorganisms possessing a large number of bioactive molecules. However, the continual and cyclic need of new antibiotics to combat the emerging resistant forms of bacterial pathogens has led to the exploration of newer econiches and biota, thereof. Marine ecosystem and its organisms, particularly the invertebrates, such as sponges, coelenterates (sea whips, sea fans and soft corals), tunicates, molluscs (nudibranchs, sea hares), echinoderms (starfish, sea cucumbers) and bryozoans (moss animals) [1,2] are recent targets of bioprospecting and mining for a large group of structurally unique natural products encompassing a wide variety of chemical classes such as terpenes, polyketides, acetogenins, peptides and alkaloids of varying structures representing and having pronounced pharmacological activities [3]. The diversity of marine organisms and the highly competitive environmental habitats in which access to space and nutrients are limited is responsible for this stunning variety.
One school of thought is that these marine invertebrates combat potential invaders, predators or competitors by producing secondary metabolites as chemical weaponry of their defence mechanisms [4]. Several of these metabolites have been characterised to be enzymes, haemolytic factors and antibiotics. In the recent years, however, investigations reveal that these bioactive factors may not be products of the marine invertebrates but may actually be produced by the microorganisms associated and/or inhabiting the sessile hosts [1,5-8]. Additionally, it is increasingly becoming evident that numerous natural products from marine invertebrates have striking structural similarities to metabolites of microbial origin. This poses a serious question on the role of host-associated microbes: Whether these are the true source of the metabolites used in defence by the host or whether these are intricately involved in biosynthesis of the metabolites used in defence [1]. Studies in these lines have demonstrated that microbes associated with invertebrates far exceed, in their bioactivity, as against that produced by free living planktonic bacteria [9,10]. In spite of this, there are limited reports on studies attempting to retrieve bacteria inhabiting marine invertebrates of Indian origin, into cultures, and scrutinising their antibacterial activity [11-13].
The Gulf of Mannar, is the world’s richest marine bioreserve lying between the southern tip of India, the south-eastern coast of Tamil Nadu state and the north-west coast of Sri Lanka. It supports a diverse and productive community of marine life. The Gulf of Mannar, is reported to harbour 295 species of sponges, 106 species of corals, 466 species of molluscs including 271 gastropods, 174 bivalves, 5 polyplacophorans, 16 cephalopods, 5 scaphopods, 100 species of echinoderms and 180 species of marine algae and seaweeds [14,15].
In view of the limited reports on the antibacterials produced by bacteria isolated from marine sponges, [11,13,16,17] corals [18,19] and bivalves [20] of Indian origin, the present study is aimed at investigating the antagonistic activities of 100 heterotrophic, halophilic bacterial bionts isolated from nine sponges (Petrosia testudinaria, Cinachyra cavernosa, Haliclona sp., Callyspongia fibrosa, Heteronema erecta, Fasciospongia cavernosa, Callyspongia reticutis var solomonensis and two unidentified sponge samples), five corals (Telesto sp., Echinogorgia reticulata, Echinomuricea indica, Echinogorgia complexa, Acropora formosa) and one bivalve (Perna viridis) collected from the Mandapam in the Gulf of Mannar. Their activity was tested against multidrug-resistant bacterial strains namely S. typhi, E. coli, P. vulgaris, A. aerogenes, S. marcesans and S. citreus.
Materials and Methods
Bacterial bionts were isolated by plating aliquots of saline macerates of tissues of sponges/corals/bivalve collected from Tamil Nadu on the South-Eastern coast of the Indian peninsula situated at (Lat. 09°19′ 37.3″N and Long. 79°10′ 20.5″E), onto nutrient-rich sterile, tryptone yeast extract agar (TYE) [21] having (g/l) MgSO4-20; CaCl2-0.2; Tryptone-5; Yeast extract-3, adjusted to pH 7, using 1 N NaOH and solidified with Agar 1.5. TYE supplemented with 3% NaCl and 25% NaCl, refered, hereafter as, (3%TYE) and (NTYE) [22-24], respectively. Colonies were purified and identified following schemes given by Smibert and Krieg, and Holt et al. [23,24] and maintained on TYE agar slopes having 3/25% NaCl. All the chemicals used were from Himedia, Mumbai, India.
Human bacterial pathogens
Salmonella typhi, E. coli, P. vulgaris, A. aerogenes, S. marcesans and S. citreus obtained from Goa Medical College, Bambolim were pregrown separately in 5 ml nutrient broth medium to an absorbance of 1 at 600 nm for 24 h at R.T.
Screening of bacterial bionts for antagonistic activity
Individual bacterial bionts were inoculated into 5 ml of 3% TYE and NTYE. After 2/7 days, aliqouts were dispensed into agar cups, borne onto Mueller Hinton agar spread plated with the individual pregrown human bacterial pathogens.
Demonstration of extracellular bioactive factor
Individual bacterial bionts were cultured in 3% TYE and NTYE at R.T. (22-28°) for a minimum of 2 days and a maximum of 7 days at 150 rpm. The cell-free supernatant obtained on separation of cells by centrifuging at 12,000×g for 20 min at 4° was extracted three times in ethyl acetate (EA), concentrated to dryness under vacuum using rotary evaporator (Buchi, Essen Germany). Crude extract was dissolved in methanol and used for bioactivity studies.
Quantification of antibacterial activity
Mueller Hinton agar plates of 3 mm thickness were seeded with the pregrown bacterial pathogens. Four milligram per litre of crude extracts was dispensed into agar cups, 6 mm in diameter borne onto the Mueller Hinton agar plates. The plates were kept standing at low temperature (4°) for 15 min, incubated at 37° and monitored for growth over a period of 24 h. Assay was carried out in triplicates and mean was recorded. Controls were maintained for each test pathogen. Each experimental data set was carried out aseptically. Zones of inhibition were measured in mms and data was computed using the earlier reported quantification procedure [25] to obtain:
Percent area specific differential antibiotic activity score (PASDAAS)= [AWG/TSA]×100 (1)
where AWG is the area on the plate without growth of test pathogen [area of zone of inhibition-area of the plug (28.26 mm2)], TSA is the total swabbed area of the pathogen on the plate (6358.5 mm2).
Percent multispecific antibiosis efficiency score (PMSAES), computed using the following equation:
PMSAES=(SPASDAASTP1-6/TPS)×100 (2)
where SPASDAASTP1-6 is the percent area specific differential antibiotic activity score of test pathogens 1-6 and TPS is total possible score for all test pathogens (i.e., 100×6=600).
Percent overall inhibition efficiency score (POIES), was calculated using the following equation:
POIES=(TNIS/TNTS)×100 (3)
where TNIS is total number of inhibited species and TNTS is total number of test species. The ideal score for multispecific inhibition would be 100.
Percent overall screening efficiency score (POSES). This is computed by,
POSES=(TPR/TAS)×100 (4)
where, TPR is the total number of positive results for each test pathogen and TAS is the total number of bionts.
Results
Hundred euryhaline bacterial bionts obtained from marine sponges, bivalve and corals were characterised on the basis of their ability to tolerate a maximum of 3 and 25% NaCl concentrations during growth in TYE medium into: Group I: Bionts growing in TYE with 3% NaCl and Group II: Bionts growing in TYE with 25% NaCl.
Culture broths of bionts growing in their respective growth media were screened for the production of extracellular bioactivity by directly exposing individual indicator cultures of bacterial pathogens to specific quantity of culture broth and observed for development of zone of inhibition of growth. The bionts giving a minimum zone of inhibition of 2 mm were considered as bioactive. Twenty percentage of Group I bionts were active against Gram-positive indicator cultures whereas 23% were active against the Gram-negative indicator cultures. On the other hand, 80% of bionts from Group II were active against Gram-positive indicator cultures and 77.27% of bionts were active against Gram-negative indicator cultures. Consequently, 46 out of 100 bionts were selected for further investigation wherein 2/7 day cell-free supernatants of active bionts were extracted in EA and used for monitoring of in vitro antibacterial activity. As seen in (Tables 1-3) extracts of bionts associated with nine different sponges, five different corals and one bivalve were active, with individual bionts having indicator culture specificity. The zones of inhibition ranged from 1-30 mm for sponge bionts, 1-20 mm for bivalve bionts and 2-30 mm for coral bionts.
| Sponges | Isolates | Zone of inhibition (mm) | Generic identity | |||||
|---|---|---|---|---|---|---|---|---|
| S. typhi | S. marcesans A. aerogens | E. coli | S. citreus P. vulgaris | |||||
| Petrosia testudinaria | GUVFPM-1 | 6 | - | 6 | - | 5 | - | Chromohalobactersp. |
| (MAM-1) | GUVFPM-2 | - | - | 2 | - | - | - | Corynebacteriumsp. |
| GUVFPM-6 | - | - | - | - | - | 2 | Pontibacillussp. | |
| Cinachyra cavernosa(MAM-2) | GUVFCCM-2 | 8 | - | 20 | 30 | 12 | 10 | Chromohalobactersp. |
| Haliclona sp.(MAM-4) | GUVFHM-2 | 7 | - | 8 | - | 7 | - | Chromohalobactersp. |
| Unidentified (MAM-5) | GUVFUM-1 | 10 | - | 8 | - | 5 | 11 | Corynebacteriumsp. |
| Callyspongia fibrosa(MAM-6) | GUVFCFM-3 | 1 | - | - | - | - | - | Corynebacteriumsp. |
| Heteronema erecta(MAM-6) | GUVFHEM-4 | - | - | - | 10 | 20 | 18 | Pseudomonas sp. |
| Callyspongia reticutis var | GUVFCM | 2 | 4 | - | - | 3 | 6 | Halobacteriasp. |
| solomonensis (NIO1) | ||||||||
| Fasciospongia cavernosa (NIO2) | GUVFFM-1 | - | 10 | 1 | 1 | - | 17 | Halobacteria sp. |
| GUVFFM-2 | Halobacteria sp. | |||||||
| Unidentified (NIO3) | GUVFSM-1 | 7 | 6 | 5 | 3 | - | 14 | Halobacteria sp. |
| GUVFSM-2 | 12 | - | 9 | -11 | 10 | - | Chromohalobacter sp. | |
Table 1: Antibacterial activity of ethyl acetate extracts of sponge bionts
| Corals | Isolates | Zone of inhibition (mm) | Generic identity | |||||
|---|---|---|---|---|---|---|---|---|
| S. typhi | S. marcesans | A. aerogens | E. coli | S. citreus | P. vulgaris | |||
| Telestosp. | GUVFTM-1 | 2 | 2 | - | - | - | Bacillus sp. | |
| (MAM-3) | GUVFTM-2 | 6 | - | 15 | 12 | 12 | 12 | Chromohalobactersp. |
| GUVFTM-3 | 2 | - | - | - | - | - | Bacillus sp. | |
| GUVFTM-4 | - | 2 | 3 | - | - | - | Bacillus sp. | |
| GUVFTM-5 | 2 | - | 2 | - | - | - | Chromohalobactersp. | |
| GUVFTM-6 | - | - | 2 | - | - | - | Chromohalobactersp. | |
| Echinogorgia | GUVFERM-1 | - | - | 2 | - | - | - | Corynebacteriumsp. |
| reticulata | GUVFERM-2 | - | - | 2 | - | - | - | Chromohalobactersp. |
| (MAM-7) | GUVFERM-7 | - | - | 8 | - | - | - | Chromohalobactersp. |
| Echinomuricea | GUVFEIM-1 | 5 | - | - | - | - | - | Chromohalobactersp. |
| indica | GUVFEIM-3 | - | - | 30 | - | - | - | Chromohalobactersp. |
| (MAM-8) | GUVFEIM-8 | - | - | 2 | - | - | - | Chromohalobactersp. |
| GUVFEIM-9 | - | - | 2 | - | - | - | Chromohlobactersp. | |
| GUVFEIM-14 | - | - | 2 | - | - | - | Psychrobactersp. | |
| Echinogorgia | GUVFECM-1 | - | - | - | 10 | 30 | - | Deinococcussp. |
| complexa | GUVFECM-2 | 2 | - | 2 | - | - | - | Chromohalobactersp. |
| (MAM-9) | GUVFECM-3 | - | - | 12 | - | - | 15 | Chromohalobactersp. |
| GUVFECM-5 | - | 6 | - | 2 | 5 | - | Chromohalobactersp. | |
| GUVFECM-6 | - | 5 | - | - | - | - | Chromohalobactersp. | |
| GUVFECM-7 | - | - | 2 | - | - | - | Bacillus sp. | |
| GUVFECM-8 | - | - | 2 | - | - | - | Virgibacillussp. | |
| GUVFECM-9 | - | - | 5 | - | - | - | Chromohalobactersp. | |
| Acrospora | GUVBAM-1 | 8 | - | 3 | - | - | - | Bacillus sp. |
| formosa | GUVBAM-2 | - | - | 2 | 13 | - | - | Pontibacillussp. |
| (GUBFM) | GUVBAM-3 | - | - | 9 | - | 5 | - | Corynebacteriumsp. |
| GUVBAM-4 | - | - | 1 | 4 | - | - | Bacillus sp. | |
| GUVBAM-5 | 7 | - | 3 | 8 | - | 8 | Unidentified | |
Table 2: Antibacterial activity of ethyl acetate extracts of coral bionts
| Bivalve | Isolates | Zone of inhibition (mm) | Generic Identity | |||||
|---|---|---|---|---|---|---|---|---|
| S. typhi | S. marcesans | A. aerogenes | E. coli | S. citreus | P. vulgaris | |||
| Pernavirdis(GUFM) GUVFPM-1 | 7 | - | 7 | 2 | - | 10 | Planococcussp. | |
| GUVFPM-2 | 20 | - | 6 | 5 | 15 | - | Bacillus sp. | |
| GUVFPM-3 | 7 | - | - | - | - | - | Bacillus sp. | |
| GUVFPM-4 | 2 | - | - | 4 | 8 | 1 | Bacillus sp. | |
| GUVFPM-5 | 3 | - | 8 | - | - | - | Psychrobactersp. | |
Table 3: Antibacterial activity of ethyl acetate extracts of bivalve bionts
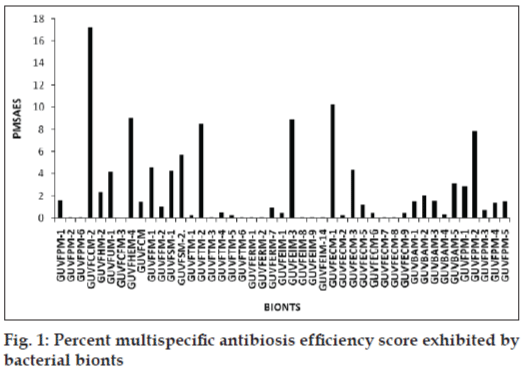
Differential antibacterial activity of individual bionts towards various indicator pathogens was deduced by comparing zone sizes of antibacterial activity and recorded as percent area specific differential antibiotic activity score (PASDAAS). Highest PASDAAS of 53.5% was shown by the sponge biont GUVFCCM-2 against E. coli and by the coral bionts namely GUVFEIM-3 and GUVFECM-1 against A. aerogenes and S. citreus, respectively. Using PASDAAS, percent multispecific antibiosis efficiency score (PMSAES) was calculated. The highest PMSAES (fig. 1) amongst the sponge bionts was shown by GUVFCCM-2, identified as Chromohalobacter sp. with a value of 17.2% (fig. 2). It was active against all the tested indicator pathogens except S. marcesans. This was followed by GUVFHEM-4 with a value of 9.08%, identified as Pseudomonas sp. GUVFHEM-1 was active against E. coli, S. citreus and P. vulgaris. Sponge bionts with PMSAES values in the range 2-6% were GUVFSM-2, with a value of 5.74%, it was identified as Chromohalobacter sp. and was active against S. typhi, A. aerogenes, E. coli and S. citreus. Biont GUVFFM-1 with a value of 4.64% was identified as Haloarchaea and was active against S. marcesans, A. aerogenes, E. coli and P. vulgaris. Bionts GUVFSM-1 identified as Haloarchaea and biont GUVFUM-1 identified as Corynebacterium sp., had near equal PMSAES values of 4.48 and 4.22%, respectively. GUVFSM-1 was active against S. typhi, S. marcesans, A. aerogenes, E. coli and P. vulgaris whereas biont GUVFUM-1 inhibited S. typhi, S. citreus, P. vulgaris and A. aerogenes. Biont GUVFHM-2, had a PMSAES value of 2.38% and was identified as Chromohalobacter sp. It was active against S. citreus, A. aerogenes and S. typhi.
Bionts having PMSAES values in the range 0.05-2% were GUVFPM-1 identified as Chromohalobacter sp., having a PMSAES value of 1.61% and was inhibitory to S. typhi, A. aerogenes and S. citreus. Biont GUVFCM with a value of 1.46% was identified as Haloarchaea and was active against P. vulgaris, S. citreus, S. marcesans and S. typhi. GUVFFM-2 identified as Haloarchaea was inhibitory against E. coli and S. marcesans and had a value of 1.03%. GUVFPM-2 and GUVFPM-6 had similar values of 0.11% and were identified as Corynebacterium sp. and Pontibacillus sp., respectively. They were active against A. aerogenes and P. vulgaris, respectively. GUVFCFM-3 had the lowest value of 0.05%, and was identified as Corynebacterium sp. and was solely active against S. typhi.
Coral biont with highest PMSAES value of 10.23% was GUVFECM-1 identified as Deinococcus sp., was active against S. typhi and A. aerogenes. This was followed by GUVFEIM-3 and GUVFTM-2 with near-equal PMSAES values of 8.91 and 8.51%, respectively. Both the isolates were identified as Chromohalobacter sp. GUVFEIM-3 was active solely active against A. aerogenes whereas GUVFTM-2 was active against S. typhi, A. aerogenes, E. coli, S. citreus and P. vulgaris. GUVFECM-3 identified as Chromohalobacter sp., was active against A. aerogenes and P. vulgaris and had a PMSAES value of 4.36%. It was followed by GUVFAM-5 with a value of 3.15%. This isolate remained unidentified as it failed to grow on repeated subculture and was active against S. typhi, A. aerogens, E. coli and P. vulgaris. Isolate with PMSAES values ranging from 1 to 2% were GUVFAM-2 identified as Pontibacillus sp., active against A. aerogenes and E. coli and had a PMSAES value of 2.03%. Near-equal PMSAES values of 1.56 and 1.5% were shown by bionts GUVFAM-3 and GUVFAM-1 against A. aerogenes and S. citreus for GUVFAM-3 and against S. typhi and A. aerogenes for GUVFAM-1. They were identified as Corynebacterium sp. and Bacillus sp., respectively. Biont GUVFECM-5 identified as Chromohalobacter sp. showed a value of 1.15% against S. marcesans, E. coli and S. citreus.
Bionts with PMSAES values in the range 0.2-1% were GUVFERM-7 at 0.91%, it was identified as Chromohalobacter sp. and inhibited A. aerogenes only. This was followed by biont GUVFTM-4 at 0.53%, identified as Bacillus sp. and active against A. aerogenes and S. marcesans. Bionts with equal values of 0.45% were GUVFEIM-1, GUVFECM-6 and GUVFECM-9. They were all identified as Chromohalobacter sp. GUVFEIM-1 inhibited S. typhi, GUVFECM-6 was active against S. marcesans whereas GUVFECM-9 inhibited A. aerogenes alone. GUVFAM-4 identified as Bacillus sp. had a value of 0.37% and was active against A. aerogenes and E. coli. GUVFTM-5, GUVFTM-1 and GUVFECM-2 had similar PMSAES values of 0.23% and were identified as, Chromohalobacter sp., Bacillus sp. and Chromohalobacter sp. and were all active against S. typhi and A. aerogenes. Bionts GUVFTM-3, GUVFTM-6, GUVFERM-1, GUVFERM-2, GUVFEIM-7, GUVFEIM-10, GUVFEIM-12, GUVFECM-8, GUVFECM-7 and GUVFECM-8 all showed similar PMSAES value of 0.11%. GUVFTM-3 inhibited S. typhi and A. aerogenes whereas the rest were active solely against A. aerogenes.
Amongst the bivalve bionts GUVFPM-2 identified as Bacillus sp. showed the highest PMSAES value at 7.8% and was active against S. typhi, A. aerogenes, E. coli and S. citreus. This was followed by biont GUVFPM-1 with a relatively lower value of 2.85%. It was identified as Bacillus sp. and was inhibitory against S. typhi, A. aerogenes, E. coli and P. vulgaris. Bionts GUVFPM-4 and GUVFPM-5 identified as Bacillus sp. and Psychrobacter sp., respectively, showed near equal PMSAES values of 1.45 and 1.5%, respectively. GUVFPM-4 inhibited S. typhi, E. coli, S. citreus and P. vulgaris whereas GUVFPM-5 was active against S. typhi and A. aerogenes. Lowest PMSAES was shown by biont GUVFPM-3 identified as Bacillus sp. with a value of 0.73% against S. typhi.
The calculated percent overall inhibition efficiency score of bionts (POIES) indicated that 33% of the bionts were active against A. aerogenes, 21% inhibited S. typhi, 14% were active against E. coli, 13% against S. citreus and 12% against P. vulgaris. The least inhibited was S. marcesans at 6%.
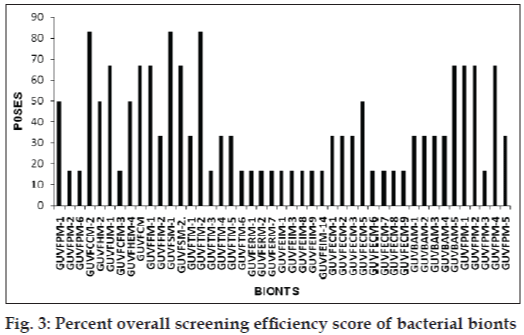
Further, the percent overall screening efficiency score of antibacterial activity (POSES) exhibited by bionts was computed by scoring presence or absence of zones by bionts against indicator cultures and depicted in fig. 3. Highest score of 83.33% was given by sponge bionts GUVFCCM-4 and GUVFSM-1 and coral biont GUVFTM-2.
Discussion
The study was an attempt to investigate the antibacterial activity of bacterial bionts from sponges, corals and bivalves thought to be involved in the epibacterial chemical defence of the host [17], in this regard, it was a noteworthy observation that 46 isolates out of the 100 screened, showed promising antibacterial activity. These bionts having activity against multidrug-resistant clinical pathogens, isolated from hospital patients, have potential to serve as drug candidates. The inhibition of the pathogens by the extracts obtained from the invertebrate-associated bacteria strongly supports the hypothesis of the microbial origin of the compounds formerly ascribed to these macro invertebrates as there are several reports on the antibacterial potential of the marine invertebrates used in this study [12,26,27].
Halophilic bacterial strains exhibited a higher antimicrobial activity against the Gram-negative bacteria than against the Gram-positive bacteria. These results are not consistent with previous studies wherein Gram-positive bacteria were more susceptible to antibiotics than Gram-negative bacteria [28]. The probable reason for this finding is that only a single Gram-positive indicator test was included in the test panel.
Our study corroborates with the findings of Chen et al. [29] in that approximately 50% of the culturable bionts exhibited antibacterial activity. The results thus confirm that invertebrate-associated microorganisms are highly potential resources of bioactive natural products [30].
The absence of antimicrobial activity in the remaining 50% bionts in the bioassays conducted does not necessarily indicate a lack of antimicrobial chemical defence, as the diffusion assay only measures cell death, however, there are reports of inhibition of other phenotypic characteristics such as chemotaxis, swarming attachment and swimming, which is also a means of counteracting bacterial invasion. Another proposed hypothesis by Geffen and Rosenberg [31] for no bioactivity of some of the isolates could be that the release of the bioactive factor is only seen following induction by deleterious microorganisms and mechanical stress which was not done in our present study. It could also be possible that the bioactive component was not extractable in the EA solvent or that it diffuses poorly in the agar medium employed. The demonstration of poor bioactivity by some bacterial isolates associated with the invertebrates is suggestive that the invertebrates resort to some other means of defence rather then production of chemical compounds as reported by Rublee et al [32].
The isolates having the greatest antimicrobial activity belonged to the genus Chromohalobacter, followed by Bacillus and Corynebacterium. The genus Bacillus have been well-known to produce lipoproteins, phenolic derivatives, aromatic acids, acetylamino acids (amino acid analogues), peptides [33], isocoumarin antibiotics and [34] bacteriocin like substances [35] having a broad antibiotic spectrum, the genus Corynebacterium is also increasingly reported as a source of bioactive agents capable of displaying competitive biosynthetic capabilities [36], however, the potential of the genus Chromohalobacter as a promising resource for antimicrobial compounds is fairly recent with no reports on the structure elucidation of the antimicrobial compound and scarce reports on its antimicrobial activity [29]. Thus, our first report on the isolation of halophilic bacterial strains from marine invertebrates as promising sources for the discovery of novel bioactive compounds is of immense importance.
The high proportion of strains producing antimicrobial compounds may be associated with an ecological role, i.e., a defensive action to maintain their niche, preventing the invasion of microbial competitors into an established microbial community. Thus, marine invertebrates represent an ecological niche harbouring a largely uncharacterised microbial community with unexploited potential sources of new secondary metabolites. Further chemical isolation and characterisation of active compounds from these bacterial extracts is under investigation, and findings will be reported in due course. As yet, there have been no published reports on the antibacterial activity of all the marine organisms discussed so far from the GoM. Thus, the present study is the first report and it proves that the EA extracts of marine bacteria associated with sponges, bivalves and corals are a promising resource having profound antibacterial activity, and thus may have potential use in medicine.
Acknowledgements
The authors are grateful to Dr. Chandra Naik, Scientist Biological Organic Chemistry Division, NIO, Goa, India for the sponge samples and keen interest in microbiological studies. The authors also express their thanks to Dr. Savio Rodrigues, Department of Microbiology, Goa Medical College, Goa, India for the clinical pathogens.
References
- Proksch P, Edrada RA, Ebel R. Drugs from the seas-Current status and microbiological implications. ApplMicrobiolBiotechnol 2002;59:125-34.
- Kijjoa A, Sawangwong P. Drugs and cosmetics from the sea. Mar Drugs 2004;2:73-82.
- Wright AE. Isolation of marine natural products. In: Cannell RJ, editor. Methods in Biotechnology, Vol. 4: Natural Products Isolation. New Jersey, USA: Humana Press Inc.; 1998. p. 365-408.
- Li KamWah H, Jhaumeer-Laulloo S, ChoongKwetYive R, Bonnard I, Banaigs B. Biological and chemical study of some soft corals and sponges collected in Mauritian waters, Western Indian Ocean. J Mar Sci 2006;5:115-21.
- Lee YK, Lee JH, Lee HK. Microbial symbiosis in marine sponges. J Microbiol 2001;39:254-64.
- Jayatilake GS, Thornton MP, Leonard AC, Grimwade JE, Baker BJ. Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J Nat Prod 1996;59:293-6.
- Mitova M, Tommonaro G, De Rosa S. A novel cyclopeptide from a bacterium associated with the marine sponge Irciniamuscarum. Z Naturforsch C 2003;58:740-5.
- Suzumura K, Yokoi T, Funatsu M, Nagai K, Tanaka K, Zhang H, et al. YM-266183 and YM-266184, novel thiopeptide antibiotics produced by Bacillus cereus isolated from a marine sponge II. Structure elucidation.J Antibiot (Tokyo) 2003;56:129-34.
- Boyd KG, Adams DR, Burgess JG. Antimicrobial and repellent activities of marine bacteria associated with algal surfaces. Biofouling 1999;14:227-36.
- Lu Y, Dong X, Liu S, Bie X. Characterization and identification of a novel marine Streptomyces sp. produced antibacterial substance. Mar Biotechnol (NY) 2009;11:717-24.
- Devi P, Wahidulla S, Kamat T, D’Souza L. Screening marine organisms for antimicrobial activity against clinical pathogens. Indian J Geo Mar Sci 2011;40:338-46.
- Rodrigues E, Supriya T, Naik CG. Antimicrobial activity of marine organisms collected off the coast of South East India. J Exp Mar BiolEcol 2004;309:121-7.
- Anand TP, Bhat AW, Shouche YS, Roy U, Siddharth J, Sarma SP. Antimicrobial activity of marine bacteria associated with sponges from the waters off the coast of South East India. Microbiol Res 2006;161:252-62.
- Available from: http://en.wikipedia.org/wiki/Gulf_of_Mannar_Marine_ National_Park. [Last cited 2012 May 9].
- Ramadhas V, Santhanam R, Venkataramani VK, Sundararaj V. Gulf of Mannar–A Profile. Tuticorin: India Fisheries College and Research Institute Publication; 1999. p. 1-35.
- Saravanakumar R, Ronald J, Ramesh U, Maheswari K. Molecular analysis of sponge associated bacteria in Gulf of Mannar Coast and their antibacterial activity against fish pathogens. Int J BiolTechnol2011;2:19-27.
- Thakur NL, Anil AC. Antibacterial activity of the sponge, Ircinia ramose: Importance of its surface-associated bacteria. J ChemEcol 2000;26:57-71.
- Chellaram C, Sreenivasan S, Anand TP, Kumaran S, Kesavan D, Priya G. Antagonistic bacteria from live corals, Tuticorin coastal waters, Southeastern India. Pak J Pharm Sci 2011;24:175-81.
- Gnanambal KM, Chellaram C, Patterson J. Isolation of antagonistic bacteria from the surface of the gorgorian corals at Tuticorin, south east coast of India. Indian J Mar Sci 2005;34:316-9.
- Chandran B, Rameshwaran G, Ravichandran S. Antimicrobial activity from the gill of Pernaviridis. Global J Biotech Biochem 2009;4:88-92.
- Steensland H, Larsen H. A study of cell envelopes of Halobacteria. J Gen 1968;55:325-36.
- Raghavan TM, Furtado I. Tolerance of an estuarine halophilicarchaebacterium to crude oil and constituent hydrocarbons. Bull Environ ContamToxicol 2000;65:725-31.
- Smibert RM, Krieg NR. Phenotypic characterization. In: Gerhardt P, editor. Methods for General and Molecular Bacteriology. Washington, DC: American Society for Microbiology; 1994, p. 607-54.
- Holt JG, Krieg NR, Sneath PH A, Staley JT, Williams ST. Bergey's Manual of Determinative Bacteriology. Baltimore: Williams and Wilkins; 1994. p. 787.
- Velho-Pereira S, Kamat NM. Antimicrobial screening of actinobacteria using a modified cross-streak method. Indian J Pharm Sci 2011;73:223-8.
- Jeyasekaran G, Jayanth K, JeyaShakila R. Isolation of marine bacteria, antagonistic to human pathogens. Indian J Mar Sci 2002;31:39-44.
- Almeida C, Kehraus S, Prudêncio M, König GM. Marilones A and B from the marine sponge derived fungus Stachylidium sp. Beilstein J Org Chem 2011;7:1636-42.
- Peláez F, Collado J, Arenal F, Basilio A, Cabello A, Matas MT, et al. Endophytic fungi from plants living on gypsum soils as a source of secondary metabolites with antimicrobial activity. Mycol Res 1998;102:755-61.
- Wang LC, Bu T, Zhang Y, Wang Y, Liu M, Lin X. Halophilic bacteria isolated from the Weihai Solar Saltern (China) phylogeneticanalysis and screening of antimicrobial and cytotoxic activities of moderately halophilic bacteria. World J MicrobiolBiotechnol 2010;26:879-88.
- Burgess JG, Jordan EM, Bregu M, Mearns-Spragg A, Boyd KG. Microbial antagonism: A neglected avenue of natural products research. J Biotechnol 1999;70:27-32.
- Geffen Y, Rosenberg E. Stress-induced rapid release of antibacterials by scleractinian corals. Mar Biol 2005;146:931-5.
- Rublee AP, Lasker RH, Gottfriend M, Roman RM. Production and bacterial colonization of mucus from the soft coral Briariumasbestinum. Bull Mar Sci 1980;30:888-93.
- Gebhardt K, Schimana J, Müller J, Fiedler HP, Kallenborn HG, Holzenkämpfer M, et al. Screening for biologically active metabolites with endosymbiotic bacilli isolated from arthropods. FEMS MicrobiolLett 2002;217:199-205.
- Pinchuk IV, Bressollier P, Sorokulova IB, Verneuil B, Urdaci MC. Amicoumacin antibiotic production and genetic diversity of Bacillussubtilisstrains isolated from different habitats. Res Microbiol2002;153:269-76.
- Bizani D, Brandelli A. Characterization of a bacteriocin produced by a newly isolated Bacillus sp. Strain 8 A. J ApplMicrobiol 2002;93:512-9.
- Zheng Z, Zeng W, Huang Y, Yang Z, Li J, Cai H, et al. Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China. FEMS MicrobiolLett 2000;188:87-91.