- Corresponding Author:
- R. Peraman
College of Pharmacy, Gulf Medical University, Ajman, UAE
E-mail: drramalingamp@gmail.com
| Date of Submission | 10 December 2014 |
| Date of Revision | 21 March 2015 |
| Date of Acceptance | 25 November 2015 |
| Indian J Pharm Sci 2015;77(6):751-757 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
By considering the current regulatory requirement for an analytical method development, a reversed phase high performance liquid chromatographic method for routine analysis of etofenamate in dosage form has been optimized using analytical quality by design approach. Unlike routine approach, the present study was initiated with understanding of quality target product profile, analytical target profile and risk assessment for method variables that affect the method response. A liquid chromatography system equipped with a C18 column (250×4.6 mm, 5 μ), a binary pump and photodiode array detector were used in this work. The experiments were conducted based on plan by central composite design, which could save time, reagents and other resources. Sigma Tech software was used to plan and analyses the experimental observations and obtain quadratic process model. The process model was used for predictive solution for retention time. The predicted data from contour diagram for retention time were verified actually and it satisfied with actual experimental data. The optimized method was achieved at 1.2 ml/min flow rate of using mobile phase composition of methanol and 0.2% triethylamine in water at 85:15, % v/v, pH adjusted to 6.5. The method was validated and verified for targeted method performances, robustness and system suitability during method transfer.
Keywords
Analytical quality by design, etofenamate, analytical target profile, liquid chromatography
Etofenamate (ETF) is a nonsteroidal antiinflammatory agent and chemically known as 2- [ [3-(trifluoromethyl) phenyl]amino]benzoic acid 2-(2-hydroxyethoxy) ethyl ester (fig. 1) [1]. It is a pale yellow viscous liquid, freely soluble in methanol and practically insoluble in water. The pKa’s of etofenamate are 6.0 and 7.0 [2]. There were few methods have been reported for the analysis of etofenamate and its degradation products in dosage forms. A high performance thin layer chromatographic (HPTLC) method [3] and stability-indicating HPLC [4] methods were reported for stability of etofenamate by forced degradation studies. Liquid chromatography and mass spectrophotometric (LC-MS) [5-7] methods were reported for quantitative analysis of etofenamate in biological samples. But detailed literature search did not reveal any cost effective and robust HPLC assay method for ETF in dosage forms. In fact stability assays are not employed in quality control for assay and content uniformity determinations, hence the need for exclusive method for assay always desirable with the advantages like less cost, shorter analysis time and robustness. Despite of few analytical methods reported for quantification of etofenamate in dosage forms or in degradation studies or in biological fluids, ‘one factor at a time’ (OFAT) based analytical methods (trial and error based on one variable) encounters lot of difficulties in optimizing robust chromatographic conditions due to various factors like limited availability of chromatographic column, solvents and chemicals and critical physicochemical properties of analyte like solubility, stability and pKa.
Recently FDA has approved few new drug applications (NDA) applying QbD approach to analytical techniques namely “Analytical Quality by Design”, like HPLC and UV spectrophotometry in which regulatory flexibility has been granted for movement within the defined method operable design region (MODR). This approach will reduce the number of out of specification (OOS) and out of trend results (OOT) hence provide base to construct six-sigma approach in pharmaceutical products. International Conference on Harmonization (ICH) Q8 (R2), ICH Q9 guidelines specified analytical target profile (ATP) for identifying MODR by analytical quality by design (AQbD) approach. ATP is a prospective summary of measurement requirements that ensure that the method is fit for the purpose where as MODR is based on multivariate approach to evaluate the effects of various method input variable on method performance or response.
Unlike usual method in literature, here first quality target product profile (QTPP) has been used to identify CQAs of the product at Table 1. Here QTPP determines the quality of the product, which helps us to define targetted responses to be optimized in life cycle of the product. Based on CQAs, Analytical target profile (ATP) was designed with accuracy, precision, specificity, linearity were selected as method performance characteristics for assay (Q-parameter). Further the method of assessment of each CQA of the product has been identified and a representative method assessment for CQA is shown in Table 2. Among the CQAs, assay, impurities and content uniformity are to be assessed by HPLC in order to satisfy the required method performance characteristics as per regulatory guidelines and hence there is a needs to establish HPLC method. This work designed on development of robust HPLC method to apply in determination ‘Assay’ component of QTPP and ATP. This type of approach is recommended in QbD based analytical methods to comply food and drug administration (FDA) and ICH Guidelines. As on today, few works have been reported based on QbD approach, but those were designed either by factorial experimental run or by chemical engineering principles [8,9]. So far, no analytical method has been reported with ATP, risk assessment and MODR concepts.
| Quality attributes | Target | Criticality |
|---|---|---|
| Dosage form | Injection | Not applicable |
| Potency | 15 mg | Not applicable |
| Physical description | Yellowish viscous liquid | Not applicable |
| Appearance | Clear and not more | Critical |
| intense than reference | ||
| Identity | Positive | Critical |
| Assay | 98.5–101.5% | Critical |
| Impurities | ≤0.1%(2,2′‑oxydiethanol) | Critical |
| Total impurity≤1.2% | ||
| Water | ≤0.5% | Not critical API does |
| not hydrolyse | ||
| Content uniformity | EP | Critical |
| Heavy metals | ≤10 ppm | Critical |
USP: United States Pharmacopeia, API: active pharmaceutical ingredient, EP: European Pharmacopoeia
Table 1: Quality target product profile for etofenamate injection
| CQA | Target | Method of assessment |
|---|---|---|
| Appearance | Clear and not more | Visual methods |
| intense than reference | ||
| Identity | Positive | IR, UV methods |
| Assay | 98.5–101.5% | HPLC (assay method), |
| UV method, HPTLC | ||
| Impurities | ≤0.1% (2,2′‑oxydiethanol) | HPLC (stability indicating |
| Total impurity ≤1.2% | method), HPTLC | |
| Content | EP | HPLC (assay method) |
| uniformity | ||
| Heavy metals | ≤10 ppm | Atomic absorption |
| spectroscopy |
Table 2: Identification of method of assessment of critical quality attributes
In accordance to FDA requirement from 2013, MODR needs to be conducted together with method validation [10,11]. But a conference on AQbD by FDA in 2014, the current (OFAT) approach in method development phase is not an appropriate method approach for routine analysis to be considered under regulatory flexibility [12,13].
In addition, consideration of analyte chemistry in AQbD is highly desired for a drug substance like etofenamate. ETF has reversed solubility profile between methanol and water that significantly affect the robustness of the LC method. The ester nature of etofenamate, and dual pka recommends the optimization of desirable pH. Hence, the pH, % aqueous phase, flow rate of mobile phase were considered as critical method variables in the experimental design. The method from this approach will have advantages such as regulatory flexibility, method with better understanding on SST and its relation to critical method input variables, high robustness and very less risk in method failure during method transfer.
Materials and Methods
All reagents used in the experimental work were of HPLC grade. HPLC grade methanol and water were purchased from Qualigens, Mumbai, India. Etofenamate ester of 99.91% purity was supplied by Uquifea, Barcelona, Spain as gift sample.
Chromatography
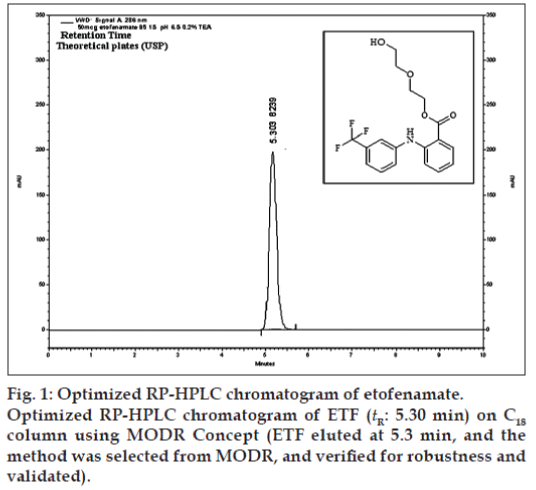
Chromatographic separations were carried out using Agilent LC system (LC-1200 series), consisting of a binary pump, a Rheodyne injector with a 20 μl loop and a photodiode array detector (DAD). A chromatographic column used was Qualisil Gold C18 (250×4.6 mm, i.d., 5 μ particle size). The output signal was monitored and processed using Ezchrome Elite software resident in a Pentium computer (Digital Equipment). Peak identify was confirmed by retention time comparison. Peak purity was assessed by purity plot. The mobile phase was composed of methanol and 0.2% v/v triethylamine (TEA) in water (pH adjusted with 10% v/v ortho phosphoric acid). The mobile phases were prepared daily, filtered through a 0.45 μ membrane filter and degassed using sonicator prior to use. The DAD detection was carried out at 286 nm wavelength, and the injection volume was 20 μl. The optimized chromatogram is shown in fig. 1.
Preparation of stock and working standard solutions of etofenamate
Stock standard solution of 0.5 mg/ml of etofenamate was prepared by accurately weighing approximately 50 mg of etofenamate (99.91% purity; density 1.317 g/cc) into a 100 ml volumetric flask and diluted to volume with mobile phase. Working solutions were prepared immediately before use by suitable dilutions of the corresponding stock solutions to appropriate concentration levels, using mobile phase as the diluents.
Preparation of sample solution
Weight equivalent to 0.5 g dosage unit of injection volume was transferred into a 50 ml volumetric flask and diluted to volume with mobile phase. The solution was filtered, then diluted immediately before use to appropriate concentration levels, using mobile phase. Detailed procedures are discussed in following sections.
Experimental design
The present AQbD work was carried out as per the cited literatures [14-16], to investigate the impact of different variables on retention time (as method response) and to verify method performances. The levels of these variables are as follows: pH (X1) of aqueous phase used in the mobile phase (5.0 and 7.0), proportion of the aqueous (X2) in the mobile phase (5% and 25%) and the flow rate (X3) of mobile phase (0.8 and 1.2 ml/min), which are given in Table 3. The retention time (Y) was used as response in experimental design as controlling response, which is expected to affect and control method responses. A 23 factorial design consisting of 3 factors at 2 levels was considered for experimental plan initially and after confirming that the process is a non linear central composite design (CCD) was used. The experimental observations along with Factorial Design (DOE) plan are shown in Table 4 and the statistical analysis is given in Table 5. The MODR was defined using all three variables. From MODR suitable method conditions were selected and subjected to verification for method performance like accuracy and precision (less than 2% RSD) and robustness as targeted response.
| Name of variables Units | −2 level −1 level 0 level 1 level 2 level | |||||
|---|---|---|---|---|---|---|
| pH of aqueous | - | 5 | 5.5 | 6 | 6.5 | 7 |
| phase | ||||||
| Percentage of | % | 5 | 10 | 15 | 20 | 25 |
| aqueous phase | ||||||
| Flow rate | ml/min | 0.8 | 0.9 | 1 | 1.1 | 1.2 |
Table 3: Levels of variables considered for doe plan
| Combination | pH of aqueous phase (X1) | Percentage of aqueous phase (X2) | Flow rate (X3) | Retention time (Y) |
|---|---|---|---|---|
| I | 5.5 | 10 | 0.9 | 4.6 |
| X1 | 6.5 | 10 | 0.9 | 5.5 |
| X2 | 5.5 | 20 | 0.9 | 11.8 |
| X1X2 | 6.5 | 20 | 0.9 | 10.1 |
| X3 | 5.5 | 10 | 1.1 | 3.4 |
| X1X3 | 6.5 | 10 | 1.1 | 4.0 |
| X2X3 | 5.5 | 20 | 1.1 | 9.1 |
| X1X2X3 | 6.5 | 20 | 1.1 | 8.2 |
| Mid points | 6 | 15 | 1 | 6.5 |
| Mid points | 6 | 15 | 1 | 6.5 |
| Mid points | 6 | 15 | 1 | 6.6 |
| Mid points | 6 | 15 | 1 | 6.6 |
| CCD | 5 | 15 | 1 | 6.7 |
| CCD | 7 | 15 | 1 | 6.7 |
| CCD | 6 | 5 | 1 | 4.1 |
| CCD | 6 | 25 | 1 | 17.8 |
| CCD | 6 | 15 | 0.8 | 8.8 |
| CCD | 6 | 15 | 1.2 | 5.8 |
CCD: Central composite design
Table 4: Central composite design plan and observed data
| Coefficient | Name of variable and Interaction | Value of coefficient | SS % | F-test | P |
|---|---|---|---|---|---|
| b0 | - | 7.025 | - | - | - |
| b1 | pH | −0.005 | 0.0003 | 0.667 | >0.1 |
| b2 | % aqueous phase | 2.56 | 86.427 | 174762 | <0.01 |
| b12 | pH and % aqueous phase | −0.4925 | 3.1988 | 6468 | <0.01 |
| b3 | Flow rate of mobile phase | −0.8675 | 9.9247 | 20068 | <0.01 |
| b13 | pH and flow rate | −0.075 | 0.0742 | 150 | <0.01 |
| b23 | % aqueous phase and flow rate | −0.125 | 0.2061 | 416 | <0.01 |
| b123 | pH, % aqueous phase and flow rate | 0.1125 | 0.1669 | 337 | <0.01 |
Error variance: 0.003, SD: 0.0183, CE: −0.5125, 95% CI of CE: From −0.548–−0.4769, nonlinear. CE: Curvature effect, CI: confidence interval, SD: standard deviation
Table 5: Analysis of observed data of 23 design
Results
Statistical analysis
The behavior of the system was explained by the following polynomial equation. Y=b0+b1X1+b2X2+b3X3+b12X1X2+b23X2X3+b13X1X3+b123X1X2X3….Eqn. 1. Where, Y is the response, b0 is the intercept, b1, b2, b3 are the regression coefficients of variables for X1, X2 and X3, respectively. b12, b23, b13 are the regression coefficients for two factor interactions between variables and b123 is the coefficient for three factor interaction between X1X2X3. Sigma Tech software was used for the statistical analysis of the experimental observations and the analysis is given in Table 5.
It can be seen from Table 6 that except for b1 i.e. coefficient of X1 (pH) all other coefficients of variables and interactions are significantly contributing at more than 99% confidence level (P<0.1). While X1(pH) has insignificant effect, X2 i.e. % aqueous phase has positive highest SS % contribution at 86%. This is the single predominant factor to control retention time. Flow rate of mobile phase X3 has negative coefficient and hence by increasing the X3 level retention time can be reduced. The strategy of optimization is to reduce X2 and increase X3 to control the retention time. ANOVA indicated that the process model with X1, X2, X3 along with interactions is highly significant at 99% Confidence level (P<0.1). Since the Curvature effect is significant and says it has nonlinear relationship between Y and Xs, it requires to go for CCD i.e. central composite design and accordingly the CCD plan and observed data are given below in Table 6. The following Quadratic model was obtained on application of SigmaTech software, Y=5.8778-0.0025X1+2.9925X2–0.8088X3– 0.4925X1X2–0.075X1X3-0.125X2X3+0.1178X12 +1.1803X22+0.2768X32…Eqn. 2.
| Range of coded values of variables | Range of absolute values of variables | Constant absolute value of variable* | Y=retention time (min) |
|---|---|---|---|
| X1=−2–2 | X1=5–7 | X3=1.2 (flow rate) | 4–6 |
| X2=−0.8–0.2 | X2=11–16 | ||
| X1=−2–2 | X1=5–7 | X2=15% (aqueous) | 5–6 |
| X3=0–2 | X3=1–1.2 | ||
| X2=−2–0.2 | X2=5–25 | X1=6.5 pH) | 4–6 |
| X3=−1.2–2 | X3=0.88–1.2 |
*Constant absolute value are used as optimized method conditions
Table 6: Summary of all contours for different method operable design region
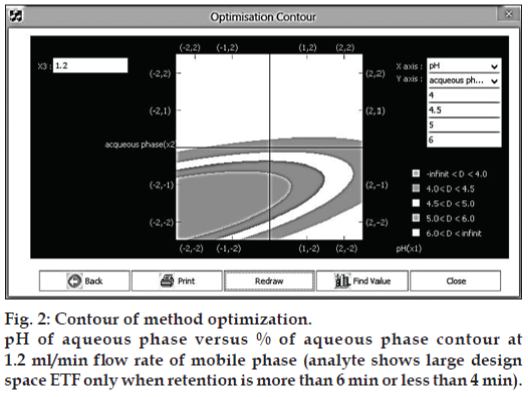
The coefficient of determination (r2) for the above process model was 0.9999. Hence the Process model is well valid to predict the behavior of the process and can be used for simulation of the process model. The design space or MODR region for robustness was achieved from contours (fig. 2). These regions offer robust processes parameters.
Contours
There could be different combinations, which may give a number of feasible solutions for robust process. X1 vs X2 with X3 as constant, X2 vs X3 with X1 as constant, X1 vs X3 with X2 as constant. Out of these combinations, which ever is the most desirable from the point of retention time that can be selected as a robust process. This contour space is called as design space in products and method operable design region (MODR) in analytical works. The MODR that control the variation in response is obtained from contours a two dimensional plot and it resembles same as fig. 2 for other variable combinations.
These three combinations are shown at Table 6. Of the three combinations, the contour (fig. 2) gave the best design space covering entire range of variables and retention time of 4 to 6 and was taken for verification purpose. It was also noted that the optimized % aqueous of 15% (X2) at flow rate 1.2 ml/min (X3), gives significant results, which are not affected by the pH from 5.5 to 6.5. Hence, the pH was kept at pH 6.5, which offered several method advantages like column long life, mobile phase stability of analyte and symmetric elution. The mathematical model the proposed contours were validated by experimental verification of predicted retention time (tR) and the results are shown in Table 7.
| Parameters | Laboratory I | Laboratory II |
|---|---|---|
| Chromatographic column | C18 (250×4.6 mm | C18 (250×4.6 mm |
| i.d, 5 µm) | i.d, 5 µm) | |
| Mobile phase* | 85% methanol: | 85% methanol: |
| 15% water (0.2% TEA; pH 6.5*) | 15% water (0.2% TEA: pH 6.5*) | |
| Flow rate* | 1.2 ml/min | 1.2 ml/min |
| Detection wavelength | 286 nm | 286 nm |
| Retention time (tR)# | 5.3 ± 0.1 min | 5.2 ± 0.1 min |
| Tailing factor | 1.12 | 1.14 |
| Theoretical plates | >8000 | >8000 |
| Repeatability (%RSD) | 0.45 | 0.52 |
| Assay (%) | 100.26 ± 0.89 | 99.89 ± 1.02 |
| (%RSD: 0.88) | (%RSD: 1.02) | |
| Precision (%RSD) | 0.63 | 0.87 |
| Robustness (precision) | 1.01% (%RSD) | |
| % aqueous (± 3%) | %RSD: 0.98 | %RSD: 1.08 |
| pH (± 0.5) | %RSD: 1.08 | %RSD: 1.26 |
| Flow rate (0.1 ml/min) | %RSD: 1.44 | %RSD: 1.31 |
*Optimized in experimental design, #Responses considered in experimental design. RSD: Relative standard deviation, TEA: triethylamine
Table 7: System suitability parameters and validation results on method transfer
Chromatographic conditions after optimization
After robust process was obtained as at fig. 1, HPLC analyses were carried out using methanol and 0.2% TEA in water (85:15, %v/v) as mobile phase, pH adjusted to 6.5 and flow rate at 1.2 ml/min on C18 analytical column, UV-PDA detection wavelength at 286 nm and 20 μl of injection volume, which gave a retention time (tR) of 5.3 min. These parameters are within MODR and hence this design space has been validated also.
Verification of method by method transfer
The robust method was verified on two instruments in different laboratory and the robustness and other system suitability parameters were compared. The % assay result and its %RSD value were calculated. Accuracy and precision were compared.
Validation of the robust method
Method parameters for robust process were obtained from MODR of contour, and verified experimentally. The verified method was validated as per ICH Q2 (R2) guidelines for assay method. Method performance like assay, precision and robustness was considered as target response.
Method validation
Method validation was performed following ICH Q2 guidelines specifications [17] for specificity, selectivity, linearity and range, accuracy, precisions, robustness, detection limit and quantitation limit.
System suitability parameters (SST)
Chromatographic conditions were tested for SST in two different laboratories. 50 μg/ml of ETF was injected in replicates through manual rheodyne injector it can be detected at retention time 5.3 min with theoretical plates more than 8000 and tailing factor of 1.12. SST parameters are within the limit in both laboratories I and II (Table 7).
Linearity
The linearity of peak area responses versus concentrations was studied from 5 to 110 mg/ml for etofenamate. A linear response was observed over the examined concentration range and the regression equation was Y=997938.46x+605166.67 (R2=0.9997) and it was good against the targeted value.
Repeatability
The system repeatability was calculated from five replicate injections of ETF at the analytical concentration of about 50 μg/ml and the %RSD found was 0.56.
Accuracy
Accuracy was studied using three different solutions, containing 90, 100 and 110 μg/ml of ETF. Recovery data are reported in Table 7. The obtained values were within the range of 99.6 and 101.3%, mean %RSD was 0.19, satisfying the acceptance criteria for the study.
Precision
Both intraday and interday precisions were studied at different levels in linearity levels are reported in Table 7. Its %RSD was within the limit (below 2%). The precision was tested for the optimized method in two different laboratories, the %RSD was below 2%.
Robustness verification
Method critical parameters such as pH, wavelength and mobile phase are considered as robustness parameters and tested on as a part of validation in laboratory I and compared with the results obtained from laboratory II. The deliberate changes in variables (Xs) were made within MODR region in order to assess the robustness of the method in same and different laboratory. % Change of organic phase was tested up to 3%. The % RSD was below 1.75% for 3% change organic phase. The results for all variables are below 2% (RSD), indicated the robustness of the method. In the same way the method was robust for all test parameters. Results are shown in Table 7.
Limit of detection and limit of quantification
LOD and LOQ were determined based on signal to noise ratio. The S/N ratio of 3:1 was taken as LOD and S/N of 10:1 was taken as LOQ. LOD was found to be 0.472 μg/ml, while LOQ was 1.416 μg/ml.
Discussion
There were few works reported [18-21] on implementation of quality by design in analytical method development. But the sequence of implementation has to be considered as per FDA. Beg et al. [19] and Kurmi et al. [20] have reported a method based on stability assay by considering resolution, as a method response to support specificity in robustness. However method verification in design space, method performance has to be added. The knowledge based QTPP for the product of etofenamate injection was constructed with the assessment of criticality for its critical attribute. Analytical target profile (ATP) was derived based on QTPP profile and then objective of this analytical QbD work was considered as assay component of QTPP of product specifications. The derived QTPP is shown in Table 1. The method assessment for attaining CQA of the product is shown in Table 2. To initiate the QbD work, the ester nature of chemical structure, pKa and solubility profile of etofenamate were considered in the selection of input variables (X1, X2, and X3) for factorial design (23). Mid points were added to find the curvature effect. Once the curvature effect was significant, CCD was adopted to get response surface to optimize design. C18 column was chosen as stationary phase due to wide acceptability pharmaceuticals and high reproducibility. In initial run, 0.2% TEA in water was chosen to eliminate the tailing effect. In this design, the concentration of TEA was not considered as quantitative variable. Because, the concentration range that is being used in chromatographic condition is very narrow. So, column temperature, organic phase, TEA components were considered as qualitative variable at 0.2% in water and were controlled. In order to achieve complete scientific understanding between method results (Y; such as tR) and input variables, a central composite design was designed and performed. The various variables and their levels were shown in Tables 3 and 4. The obtained experimental results was subjected various statistical parameter for better understanding and was found to be a nonlinear relationship between input variable and response. The statistical data and ANOVA analysis are shown in Table 5. The curvature effect was significant, so the Quadratic model (Eqn. 2) was obtained using of Sigma Tech software.
The above model was validated by coefficient of determination (R2). The value was 1.00 indicated the process model is valid for predicting the behavior of the process and it was used for simulation of the process model and contours were obtained. The design space or MODR region for robustness was achieved from contours. These regions offer robust processes parameters and shown in Table 7. The obtained method conditions and chromatograms are shown in fig. 1.
In the optimized model pH was chosen 6.5 indicated the suitability for ester in column because lower pH or even pH 7.0 may hydrolyze etofenamate in sample or in column. The flow rate was optimized at 1.2 ml/min, it’s required as % organic is higher. The method was validated for accuracy and precision during method transfer between laboratory I and II (Table 7). The result was satisfactory.
The AQbD approach on development of reversed phase high-performance liquid chromatographic method for etofenamate in pharmaceutical dosage forms. The prediction form MODR has been verified by actual experimental results indicating its robustness. Thus the method developed based on AQbD is more precise, accurate, and robust during method transfer and also cost effective. This method satisfy the design space concept for analytical method (MODR) and suitable for regulatory submission under regulatory flexibility.
Acknowledgments:
The authors wish to thank the UQUIFEA, Barcelona, Spain and Sigma Tech Services private limited, Hyderabad, India for supporting this work. This work was conducted at Research laboratory of Raghavendra Institute of Pharmaceutical Education and Research, Andhra Pradesh, India in collaboration with Sigma Tech. Consultancy, Hyderabad, India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Chlud K. Use of topical nonsteroidalantiinflammatory drugs in aggravated and decompensated arthroses. Wien Med Wochenschr 1999;149:546-7.
- The European Pharmacopoeia Commission. General Notices. European Pharmacopoeia. 7th ed. Strasbourg: Council of Europe; 2011. p. 1561-2.
- Beckermann B, Bock E, Kamp R, Dell HD. Plasma level studies in volunteers after intramuscular administration of various doses of etofenamate in oily solutions. Arzneimittelforschung 1990;40:305-11.
- Ramalingam P, Devanna N, Theja DH, Sudhakara K. Development and validation of stability-indicating assay of etofenamate by RP-HPLC and characterization of degradation products. Sci Pharm 2013;81:1017-28.
- Mamta PD, Anandan P, Arindam MV. Development of rapid and sensitive method for estimation of etofenamate in human plasma by LCMS/MS. Int J ApplBiol Pharm Technol 2011;2:323-7.
- Negoro T, Nakao M, Kagemoto A, Hoshimoto M. Synthesis of carboxyl carbon14 labeledetofenamate and its major metabolite. Radioisotopes 1982;31:636-40.
- Dannhard G, Laufer S, Lehr M. HPLC determination of eofenamate and flufenamic acid in biological material. ClinChem 1988;34:2580-1.
- Karmakar S, Garbar R, Genchank Y, George S, Yang X, Hammond R. Quality by design (QbD) based development of stability indicating HPLC method for drug and impurities. J ChromatogrSci 2011;49:439-46.
- Hockman KK, Berengut D. Determination of pharmaceutical excipients by design of experiments in chemical engineering. Chromatogr Res Int 1985;102:142-8.
- International Conference on Harmonization (ICH). Tripartite Guidelines, ICH Q8 (R2): Pharmaceutical Development; 2009.
- International Conference on Harmonization (ICH). Tripartite Guidelines, ICH Q9: Quality Risk Management; 2006.
- Chatterjee S. QbD Consideration for Analytical Methods – FDA Perspectives, US IFPAC Annual Meeting; Baltimore; 2013.
- Alexander Schmidt H. Validation and Transfer, Berlin, 4th Annual Conference on Analytical Method Development, Germany; 2014.
- Lewis GA, Mathieu D, Phan Tan L. Pharmaceutical Experimental Design. New York: Marcel Dekker; 1999.
- Wen-Ying H, Pei-Chi L, Ling-Kuei H, Li-Ping L, Wayne CL. Stability studies of ascorbic acid 2-glucoside in cosmetic lotion using surface response methodology. Bioorg Med ChemLett 2013;23:1583-7.
- Srinubabu G, Raju CH, Sarath N, Kiran Kumar P, SeshagiriRao JV. Development and validation of a HPLC method for the determination of voriconazole in pharmaceutical formulation using an experimental design. Talanta 2007;71:1424-9.
- International Conference on Hormonization (ICH), Tripartite Guidelines, ICH Q2 (R1): Validation of Analytical Procedures: Text and Methodology, London; 2009.
- Orlandini S, Pinzauti S, Furlanetto S. Application of quality by design to the development of analytical separation methods. Anal BioanalChem 2013;405:443-50.
- Beg S, Sharma G, Katare OP, Lohan S, Singh B. Development and validation of stability-indicating liquid chromatographic method for estimating olmesartanmedoxomil using quality by design. J ChromatogrSci 2015:53;1048-59.
- Kurmi M, Kumar S, Singh B, Singh S. Implementation of design of experiments for optimization of forced degradation conditions and development of a stability-indicating method for furosemide. J Pharm Biomed Anal 2014;96:135-43.
- Ramalingam P, Kalva B, Yiragamreddy PR. Analytical quality by design: A tool for regulatory flexibility and robust analytics. Int J Anal Chem 2015;2015:11-9.