- *Corresponding Author:
- Shajidul Karim
Department of Pharmaceutical Analysis and Quality Assurance, Himalayan Pharmacy Institute, Majitar, Sikkim 737136, India
E-mail: shajidulkarim451@gmail.com
| Date of Received | 10 March 2020 |
| Date of Revision | 6 March 2023 |
| Date of Acceptance | 8 August 2024 |
| Indian J Pharm Sci 2024;86(4):1261-1267 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Evogliptin is an anti-diabetic drug, which comes under the class of gliptin derivatives for the inhibition of selective dipeptidyl peptidase-4 inhibitor. The developed ultraviolet spectrophotometric method was simple, sensitive, accurate, precise and economic for the development and validation of evogliptin in bulk and tablet dosage form. In this present study, the analytical method validation and development of evogliptin was done using the different parameters of method validation as per International Council for Harmonisation Q2(R1) guidelines. Water using as a solvent and it shows the maximum wavelength at 266 nm and then performed all the parameters of analytical method validation like accuracy, precision, linearity, range, robustness, ruggedness, limit of detection and limit of quantitation. Evogliptin showed linearity over the range of 2-48 µg/ml. The correlation coefficient value obtained was 0.996 with the regression equation y=0.0032x+0.0005. The accuracy studies was done in spiking method and the recoveries ranging from 97.07 %-106.13 %. The percentage relative standard deviation for intra-day precision was 0.44 and inter-day precision was 0.59. The limit of detection was 1.1 µg/ml and limit of quantitation was 3.33 µg/ml respectively. The method has shown good and consistent recoveries and is validated as per International Council for Harmonisation guidelines and can be used for routine quality control analysis of evogliptin in dosage form.
Keywords
Dipeptidyl peptidase-4 inhibitor, ultraviolet spectrophotometric method, method development, validation, percentage relative standard deviation
Evogliptin is an anti-diabetic drug, which comes under the class of gliptin derivatives for the inhibition of selective Dipeptidyl Peptidase-4 (DPP-4) inhibitor. It was first approved in South Korea for treating the patient of type 2 diabetes for lowering of blood glucose [1]. It was taken orally by the dose of 5 mg once daily without regard to food [2]. In the structure of evogliptin, it contains a small molecule of piperazine derivative that potently inhibits DPP-4 with high selectivity. DPP-4 is the enzyme responsible for rapidly degrading the incretin hormones Glucose-dependent Insulinotropic Polypeptide (GIP) and Glucagon-Like-Peptide (GLP-1). GIP and GLP-1 are the two primary incretin hormones secreted from K and L cells, respectively [3]. In gastrointestinal tract, its response to the ingestion of nutrients, they stimulate insulin secretion from pancreatic β-cells. GLP-1, which also suppresses glucagon secretion, promotes hepatic glycogen storage and slows gastric emptying, among other actions. Thus, inhibition of DPP-4 increases the plasma levels of GLP-1, promotes insulin secretion and reduces blood glucose levels. International Union of Pure and Applied Chemistry name of evogliptin is (3R)-4- [(3R)-3-amino-4-(2,4,5-triflurophenyl)butanoyl]-3- [(tert-butoxy)methyl]piperazin-2-one, which have the chemical formula of C19H26F3N3O3 and molecular weight of 401.43 (fig. 1) [4-8].
While reviewing of literature for analytical method of validation it was observed that till now there are no such literature found about the method validation of evogliptin. Hence the present research work is based on the analytical method development and validation of evogliptin in bulk dosage form by Ultraviolet (UV)-spectrophotometric method. The objective of this research is to develop and validate the analytical method validation of evogliptin in bulk dosage form by UV-spectrophotometry by studying different parameters as per International Council for Harmonisation (ICH) Q2R1 guidelines.
Materials and Methods
Instrumentation and chemicals:
The instrument employed in this research work is UV-visible spectrophotometer made by Shimadzu (UV-1800), evogliptin (Alkem laboratories, India), distilled water. On the basis of their solubility study, water is selected as a solvent system.
Determination of wavelength of maximum absorption (λmax) [9-11]:
A standard stock solution of evogliptin was prepared using water as solvent. Then, the reference solution was scanned in the wavelength region of 190-400 nm.
Preparation of standard stock solution:
1 mg of evogliptin was weighed out and transferred in a 10 ml volumetric flask, dissolved with water and made up the volume with the same. Then 1 ml was taken out from this solution and again diluted up to 10 ml with the same solvent to get 10 µg/ml solution of evogliptin standard.
Preparation of standard sample solution:
Average weight of 10 marketed evogliptin tablets was taken and recorded. Weight equivalent to 1 mg of evogliptin tablet was taken and mixed with water in a 10 ml volumetric flask and made the volume up to the mark with the same solvent and filter it. Then 1 ml of filtrate solution was taken out from this solution and diluted upto 10 ml with the same solvent in a 10 ml volumetric flask to get a concentration of 10 µg/ml. Both the solution was taken to UV-spectrophotometer for further analysis.
Method validation parameters [12]:
Linearity and range: Six solutions of different concentration were prepared from the standard stock solution of evogliptin for linearity study. The absorbance of these solutions was observed and water as blank at 266 nm and the obtained data was used for linearity calibration curve.
Accuracy: Accuracy of the developed method was carried out by performing recovery studies using standard addition method, in which standard drug was added in three different concentrations (50 %, 75 % and 100 %) to the pre-analysed formulation (10 µg/ml). The result of recovery studies is expressed in percentage Relative Standard Deviation (% RSD).
Precision: Precision of the method was performed by intra-day and inter-day variation studies. The intra-day precision and inter-day precision was ascertained by determining absorbance of six replicates of the fixed concentration of the drug 10 µg/ml at six different time period of the same day and on six different days. The result of precision studies is expressed in % RSD.
Limit of Detection (LOD) and Limit of Quantitation (LOQ): The LOD and LOQ was determined with the help of linearity curve, for the assay was calculated using the following formula.
LOD=3.3×(standard deviation of y-intercept of the regression line/slope of the calibration curve)
LOQ=10×(standard deviation of y-intercept of the regression line/slope of the calibration curve)
Robustness and Ruggedness: Robustness of the method was determined by measuring the absorbance of 10 µg/ml solution of evogliptin at 263 nm, 266 nm and 269 nm. Ruggedness of the method was determined on carrying out the method by two different analysts.
Results and Discussion
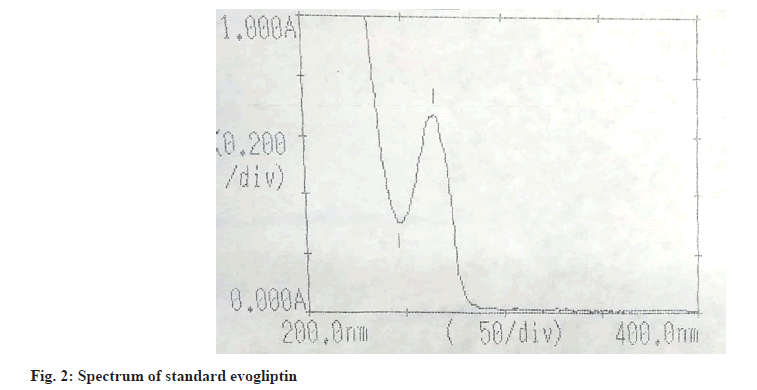
During the method developmental stage, the drugs were scanned in the UV range of 190-400 nm to observe their respective λmax i.e., the wavelength at which the drug shows maximum absorbance in their respective spectra. From the spectra, evogliptin showed absorbance maxima at 266 nm (fig. 2).
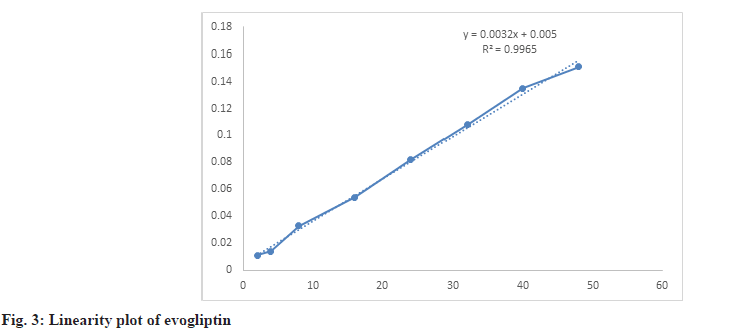
Evogliptin showed linearity over the range of 2-48 µg/ml. The correlation coefficient value obtained was 0.996 with the regression equation y=0.0032x+0.005 (Table 1 and fig. 3). Inter-day and Intra-day precision studies were carried out by taking six replicates sample. Values of percentage RSD for intra-day precision were 0.44. Similarly, for inter-day precision percentage RSD was found to be 0.59 (Table 2 and Table 3).
| Parameters | Evogliptin |
|---|---|
| Linearity | 2-48 µg/ml |
| Slope | 0.003 |
| Intercept | 0.005 |
| Correlation coefficient | 0.996 |
Table 1: Regression and Characteristics of Evogliptin
| Parameters | Evogliptin |
|---|---|
| % purity | 100.16 % |
| 100.51 % | |
| 100.47 % | |
| 101.20 % | |
| 100.83 % | |
| 101.33 % | |
| Mean | 100.75 % |
| Standard deviation | 0.45 % |
| RSD | 0.44 % |
Table 2: Intra-Day Precision Data
Recovery study was performed by standard spiking method, with view to justify the accuracy of proposed method. The experiment was performed in triplicate percentage recovery, mean percentage recovery was calculated for each concentration. The method has good and consistent recoveries ranging from 97.07 %-106.13 % (Table 4). Robustness was determined by increasing and decreasing the λmax of the sample and the experiment was performed in triplicate and percentage RSD was calculated for each λmax (Table 5). Ruggedness was determined by estimation of drug by different analyst using same procedure in different days and the experiment was performed in triplicate and the percentage RSD shows the good ruggedness of the given method (Table 6). LOD and LOQ was calculated using formula and calibration curve and the standard deviation was calculated by the response of blank. The LOD was 1.1 µg/ml and LOQ was 3.3 µg/ml respectively (Table 7).
| Parameters | Evogliptin |
|---|---|
| % purity | 101.43 % |
| 99.87 % | |
| 100.11 % | |
| 101.40 % | |
| 101.37 % | |
| 100.77 % | |
| Mean | 100.95 % |
| Standard deviation | 0.60 % |
| RSD | 0.59 % |
Table 3: Inter-Day Precision
| Drug | Concentration of sample (µg/ml) | Concentration of standard added (µg/ml) | Recovery % | Mean % recovery (n=3) |
|---|---|---|---|---|
| Evogliptin | 10 | 5 | 100.0 | 99.98 |
| 89.47 | ||||
| 110.52 | ||||
| 10 | 7.5 | 101.75 | 97.07 | |
| 94.73 | ||||
| 94.73 | ||||
| 10 | 10 | 107.89 | 106.13 | |
| 102.63 | ||||
| 107.89 |
Table 4: Accuracy Data
| Wavelength | 263 nm | 266 nm | 269 nm |
|---|---|---|---|
| Mean (% purity) | 105.23 | 97.28 | 102.27 |
| Standard deviation | 1.03 | 1.58 | 1.57 |
| % RSD | 0.97 | 1.62 | 1.53 |
Table 5: Robustness Data
| Parameter | Analyst I | Analyst II |
|---|---|---|
| Mean | 99.99 % | 99.95 |
| Standard deviation | 2.52 | 0.6 |
| % RSD | 2.52 | 0.6 |
Table 6: Ruggedness Data of both the Analyst
| Parameters | Evogliptin |
|---|---|
| Slope | 0.003 |
| Standard deviation of response | 0.001 |
| LOD | 1.1 µg/ml |
| LOQ | 3.33 µg/ml |
Table 7: LOD and LOQ Data
After the literature survey, there are no previous works on the analytical method validation of evogliptin in pharmaceutical dosage form by UV-spectrometric method, so this method is developed in according to the ICH Q2R1 guidelines. In the present work evogliptin showed good solubility in water, the spectral analysis showed the λmax of evogliptin as 266 nm when water as the solvent system. The calibration curve or linearity was obtained for series of concentration range of 2-48 µg/ml and a linear relationship response was obtained when the absorbance was plot against concentration, the calibration curve for evogliptin drug was found to be linear and hence suitable for estimation of the drug. Based on their linearity or standard curve, the assay concentration was chosen as 10 µg/ml [13]. The proposed method was validated for linearity, range, accuracy, precision, robustness, ruggedness, LOD and LOQ as per ICH guidelines. The accuracy was measured in spiking method which was determined by three different concentration like 50 %, 75 % and 100 % and the method has good and consistent recoveries ranging from 97.07 %-106.13 %, which shows that the percentage recoveries of evogliptin is good as compare to the other class of gliptin derivatives. The precision was measured in repeatably like intra-day and inter-day precision. The percentage RSD for evogliptin were 0.44 and 0.59 for intra-day and inter-day precision respectively, which was performed under the lab and in different days and different times, the precision results which are in the acceptable limit and as the less value suggest that the method is precise. The results of robustness were performed in triplicate sample in some variation or different wavelength and it shows as good % RSD value and it suggest that the sample can be detected in the upper and lower range of the wavelength of evogliptin and as the value of percentage is less than 2 so the method is robust. Ruggedness was found to be 2.54 and 0.60 for the analyst I and analyst II, the robustness result shows that the changes of analyst which changes the assay result. The LOD was 1.1 µg/ml and LOQ was 3.33 µg/ml respectively, as the result shows that the instrument can detect and quantified only at this amount, which shows that the method is good and reliable. All these results show that the percentage purity or assay value of the Evogliptin in pharmaceutical dosage with its label claim shows that its good assessment of the product. The method was validated and according to the other class of gliptin derivatives because no one has been working previously on this drug, so this was validated [14]. Hence the analytical method for evogliptin in pharmaceutical dosage form was validated as per ICH guidelines [15].
In conclusion, the objective of the present work was to develop and validate the analytical method validation of evogliptin in bulk dosage form by UV-spectrophotometry in different parameter and to validate the method as per ICH guidelines. The developed UV spectrophotometric method was simple, sensitive, accurate, precise and economic and cost of materials and labours. The method has shown good and consistent recoveries and is validated as per ICH guidelines and can be used for routine quality control analysis of evogliptin in dosage form.
Acknowledgements:
Authors are grateful to Department of Pharmaceutical Analysis and Quality Assurance, Himalayan Pharmacy Institute, Sikkim for providing the facility to carry out the work on UV-spectrophotometry is highly acknowledged. Author would like to thank Dr. Biswajit Dash for guiding me for the research work.
Conflict of interest:
The authors declare no conflict of interest in this work.
References
- McCormack PL. Evogliptin: First global approval. Drugs 2015;75:2045-9.
[Crossref] [Google Scholar] [PubMed]
- Tan X, Hu J. Evogliptin: A new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother 2016;17(9):1285-93.
[Crossref] [Google Scholar] [PubMed]
- Kim HJ, Kwak WY, Min JP, Lee JY, Yoon TH, Kim HD, et al. Discovery of DA-1229: A potent, long acting dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem Lett 2011;21(12):3809-12.
[Crossref] [Google Scholar] [PubMed]
- Kim MK, Chae YN, Kim HD, Yang EK, Cho EJ, Choi SH, et al. DA-1229, a novel and potent DPP4 inhibitor, improves insulin resistance and delays the onset of diabetes. Life Sci 2012;90(1-2):21-9.
[Crossref] [Google Scholar] [PubMed]
- Gu N, Park MK, Kim TE, Bahng MY, Lim KS, Cho SH, et al. Multiple-dose pharmacokinetics and pharmacodynamics of evogliptin (DA-1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des Dev Ther 2014:1709-21.
[Crossref] [Google Scholar] [PubMed]
- Kim TE, Lim KS, Park MK, Yoon SH, Cho JY, Shin SG, et al. Evaluation of the pharmacokinetics, food effect, pharmacodynamics, and tolerability of DA-1229, a dipeptidyl peptidase IV inhibitor, in healthy volunteers: First-in-human study. Clin Ther 2012;34(9):1986-98.
[Crossref] [Google Scholar] [PubMed]
- Lee DY, Kim JH, Shim HJ, Jeong HU, Lee HS. Absorption, metabolism, and excretion of [14C] evogliptin tartrate in male rats and dogs. J Toxicol Environ Health A 2018;81(11):453-64.
[Crossref] [Google Scholar] [PubMed]
- Ajmani AK, Agrawal A, Prasad BL, Basu I, Shembalkar J, Manikanth N, et al. Efficacy and safety of evogliptin versus sitagliptin as an add-on therapy in Indian patients with type 2 diabetes mellitus inadequately controlled with metformin: A 24-week randomized, double-blind, non-inferiority, EVOLUTION INDIA study. Diabetes Res Clin Pract 2019;157:107860.
[Crossref] [Google Scholar] [PubMed]
- Reichal CR, Rao MG. Development and validation of spectrophotometric method for simultaneous estimation of gliclazide and sitagliptin phosphate monohydrate in bulk and pharmaceutical dosage form. Int J Pharm Pharm Sci 2015;7:372-6.
- Jayaprakash R, Natesan SK, Lalitha K. Stability indicating RP-HPLC method development and validation for the simultaneous determination of vildagliptin and metformin in pharmaceutical dosage form. Int J Pharm Pharm Sci 2017;9(3):150-7.
- Zope MV, Patel RM, Patel A, Patel SG. Development and validation of a stability indicating rp-hplc method for the determination of potential degradation products of difluprednate in ophthalmic emulsion. Int J Pharm Pharm Sci 2018;10:79-86.
- Guideline IH. Validation of analytical procedures: Text and methodology Q2 (R1). 2005;1(20):5.
- Barwick V. Preparation of calibration curves: A guide to best practice; 2013.
- Pathade P, Imran M, Bairagi V, Ahire Y. Development and validation of stability indicating UV spectrophotometric method for the estimation of sitagliptin phosphate in bulk and tablet dosage form. J Pharm Res 2011;4(3):871-3.
- Himabindu T, Narmadha S, Sireesha D, Vasudha B. Development and validation of spectrophotometric method for the simultaneous estimation of metformin hydrochloride and sitagliptin phosphate in tablet dosage form. World J Pharm Res 2016;5(7):1011-8.