- *Corresponding Author:
- J. Y. Zhao
Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Heilongjiang 150001, Harbin, china
E-mail: zjyvipn@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of BiopharmaceuticalSciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “199-204” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study focuses to find out the influence of atorvastatin calcium with ezetimibe on the effectiveness, safety and blood lipid levels in coronary heart disease. 95 coronary heart disease individuals admitted between April 2020 and April 2023 were selected and were assigned as control (n=45) and research group (n=50). Control group received atorvastatin calcium monotherapy and the research group were given a combination of atorvastatin calcium and ezetimibe. The effectiveness and adverse effects like gastrointestinal reactions, myalgia, loss of appetite and rash were comparatively analyzed. Blood lipid levels (low and high-density lipoprotein cholesterol, total cholesterol and triglyceride) were evaluated. Further, serum inflammatory indices namely, tumor necrosis factor-alpha, interleukin-6 and C-reactive protein were analyzed. Similarly oxidative stress indices (myeloperoxidase, malonyldialdehyde and superoxide dismutase) were compared between both the groups. The research group was found to have an evidently higher total effective rate and a similar incidence of total adverse reactions with the control group. Marked reduction in low density lipoprotein cholesterol, total cholesterol, triglyceride, tumor necrosis factor-alpha, interleukin-6, C-reactive protein, myeloperoxidase and malonyldialdehyde in the research group after treatment was found; while an evident increase in high-density lipoprotein cholesterol and superoxide dismutase were identified, which was higher than the baseline and the control group. Conclusively, atorvastatin calcium plus ezetimibe is effective and safe in treating coronary heart disease, and can significantly ameliorate blood lipid levels, serum inflammatory and oxidative stress indices.
Keywords
Atorvastatin calcium, ezetimibe, coronary heart disease, myeloperoxidase, malonyldialdehyde
As a fatal disease worldwide, Coronary Heart Disease (CHD) not only affects patients’ quality of life, but it also causes heavy social and medical costs[1,2]. Its etiology is related to atherosclerotic plaqueinduced coronary artery blood flow restriction and obstruction[3]. The major therapeutic strategies for CHD are coronary artery bypass, thrombolytic therapy and Percutaneous Coronary Intervention (PCI). Despite the effectiveness of these treatment strategies, some patients may still experience disease recurrence or progression, resulting in poor prognosis[4,5]. Hence, it is urgent to continue to explore and optimize the clinical treatment of CHD for improving patient related outcomes.
Statins can be used as the primary treatment option for secondary prevention of CHD as well as for primary prevention of high-risk CHD[6]. Atorvastatin Calcium (AC), the most commonly utilized statin helps to prevent CHD, exerts its lipid regulation effect by selectively inhibiting 3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG-CoA) reductase in vivo[7]. However, AC alone may cause drug resistance in some patients and thus affecting the effect of blood lipid regulation[8]. Ezetimibe (EZE), which is a nonstatin lipid-modifying drug, is a selective inhibitor of intestinal cholesterol absorption, which can play a good role in blood lipid regulation while taking into account good safety. Its blood lipid regulation is associated with blocking of intestinal absorption (diet, biliary cholesterol and related phytosterols), as well as its ability to inhibit cholesterol, phytosterol intake and absorption by intestinal cells through binding to Niemann-Pick C1-Like 1 (NPC1L1) protein[9].
Currently, there are few assessments on the effects of AC combined with EZE on the effectiveness, safety and blood lipid levels in CHD patients. Based on these assessments, this study investigates the perspective of serum inflammation and Oxidative Stress (OS), in order to make a contribution to the exploration of treatment strategies for patients with CHD.
Materials and Methods
General information:
95 CHD research individuals admitted to The First Affiliated Hospital of Harbin Medical University from April 2020 to April 2023 were considered for this study. Among them, 45 individuals were assigned to be control group and the remaining 50 individuals were considered to be research group. The control group (n=45) was given AC alone while the research group (n=50) were given AC combined with EZE. Both the groups were clinically comparable with no statistical inter-group difference in general data (p>0.05). Informed consent was obtained from each patient after the study was ratified by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University.
Inclusion criteria:
All the patients who met the diagnostic criteria for CHD established by the American College of Cardiology (ACC) those who agreed to cooperate with the study; patients who did not use anti-inflammatory or lipid-regulating drugs recently and those patients who had no allergic reactions to the drugs used in the study, i.e., AC and EZE were included in this study.
Exclusion criteria:
Patients having malignant tumor and severe dysfunction of other organs; patients with autoimmune defects or coagulation dysfunction and patients with acute infection, mental illness and cognitive dysfunction were excluded from the study.
Methods:
The control group patients were given 20 mg of AC tablets (Beijing Kangruina Biotechnology Co., Ltd., no: B10275) daily for 1 mo. Similarly, on this basis the research group was given 10 mg of EZE (Beijing Kangruina Biotechnology Co., Ltd., LA2728) along with AC tablets (as per the dosage mentioned above) orally, daily for 1 mo.
Outcome measures:
The two groups were comparatively analyzed in terms of drug effectiveness and safety by evaluating the adverse effects such as Gastrointestinal Reactions (GIRs), myalgia, loss of appetite, and rashes. Further, blood lipid levels like Low Density Lipoprotein- Cholesterol (LDL-C) and High (H) HDL-C, Total Cholesterol (TC) and Triglyceride (TG) were assessed. Subsequently, the serum inflammatory markers such as Tumor Necrosis Factor-Alpha (TNF)-α, Interleukin (IL)-6, C-Reactive Protein (CRP). Ultimately, OS indices like Myeloperoxidase (MPO), Malonyldialdehyde (MDA) and Superoxide Dismutase (SOD) were also analyzed.
Efficacy: Drug efficacy was clinically evaluated using grades like markedly effective, effective and ineffective. Markedly effective corresponds to the significant improvement of blood lipid levels after treatment, disappearance of all clinical symptoms and the absence of angina pectoris within 30 d after treatment. If the blood lipid levels seem to be improved after treatment, the clinical symptoms are significantly improved and the frequency of angina pectoris is <80 % within 30 d after treatment, it is considered as effective. Failure to meet the criteria of either markedly effective or effective, then it is considered as ineffective. Total effective rate=(markedly effective+effective) cases/total cases
Safety: Drug safety was determined by examining the incidence of adverse effects after drug treatment. The incidence of GIRs, myalgia, loss of appetite, rash and other adverse reactions after treatment between the two groups were analyzed and recorded.
Blood lipid levels: Blood samples were collected from all patients before and after treatment. The collected samples were centrifuged and the serum obtained was detected for indices such as LDL-C, HDL-C, TC and TG using an automatic biochemical analyzer. The observed values were recorded.
Serum inflammatory markers: TNF-α, IL-6 and CRP in patient’s serum before and after treatment were measured using Enzyme-Linked Immunosorbent Assay (ELISA).
OS: The pre- and post-treatment values of MPO and MDA were measured using immunoturbidimetry and similarly SOD was determined by electrochemiluminescence.
Statistical analysis:
Continuous variables were represented by mean±Standard Deviation (x±SD); independent samples followed student’s t-test and the paired t-test for comparisons before and after treatment within groups, respectively. Chi-square (χ2) test was performed to identify the inter-group differences of categorical variables expressed by n (%). In this study, Statistical Package of Social Sciences (SPSS) version 22.0 software was used for analyzing the collected data where p<0.05 was considered to be statistically significant.
Results and Discussion
Primarily base-line data like age, gender, disease course, weight, and presence and absence of diabetes, hypertension and cerebral infarction were analyzed. There were no notable differences identified between groups (p>0.05) (Table 1).
| Characteristics | Control group (n=45) | Research group (n=50) | χ2/t | p |
|---|---|---|---|---|
| Age (y) | 61.82±6.25 | 64.40±7.92 | 0.084 | 1.749 |
| Gender (male/female) | 29/16 | 28/22 | 0.704 | 0.402 |
| Disease course (mo) | 44.2±7.77 | 45.82±10.04 | 0.872 | 0.385 |
| Weight (kg) | 66.76±4.92 | 65.64±6.51 | 0.938 | 0.351 |
| Diabetes mellitus (with/without) | 14/31 | 16/34 | 0.009 | 0.926 |
| Hypertension (with/without) | 12/33 | 10/40 | 0.592 | 0.442 |
| Cerebral infarction (with/without) | 16/29 | 20/30 | 0.199 | 0.656 |
Table 1: General information of patients of the two groups
The influence of AC+EZE on curative effectiveness in CHD was observed between the two groups. The total effective rates of the research and control groups were 92.00 % and 75.56 % respectively. These results indicated higher effectiveness of AC+EZE therapy when compared with AC monotherapy (p<0.05) (Table 2).
| Efficacy | Control group (n=45) | Research group (n=50) | χ2 | p |
|---|---|---|---|---|
| Markedly effective | 14 (31.11) | 25 (50.00) | ||
| Effective | 20 (44.44) | 21 (42.00) | ||
| Ineffective | 11 (24.44) | 4 (8.00) | ||
| Total effectiveness | 34 (75.56) | 46 (92.00) | 4.817 | 0.028 |
Table 2: Analysis of the effect of AC+EZE on the curative effect
Analysis of medication safety of AC+EZE in treating CHD was carried out. The two groups had an equivalent number of patients having GIRs, myalgia, loss of appetite and rashes (p>0.05) (Table 3).
| Adverse effects | Control group (n=45) | Research group (n=50) | χ2 | p |
|---|---|---|---|---|
| GIRs | 1 (2.22) | 2 (4.00) | ||
| Myalgia | 1 (2.22) | 1 (2.00) | ||
| Loss of appetite | 3 (6.67) | 2 (4.00) | ||
| Rash | 2 (4.44) | 1 (2.00) | ||
| Total | 7 (15.56) | 6 (12.00) | 0.234 | 0.615 |
Table 3: Analysis of medication safety of AC+EZE in treating CHD
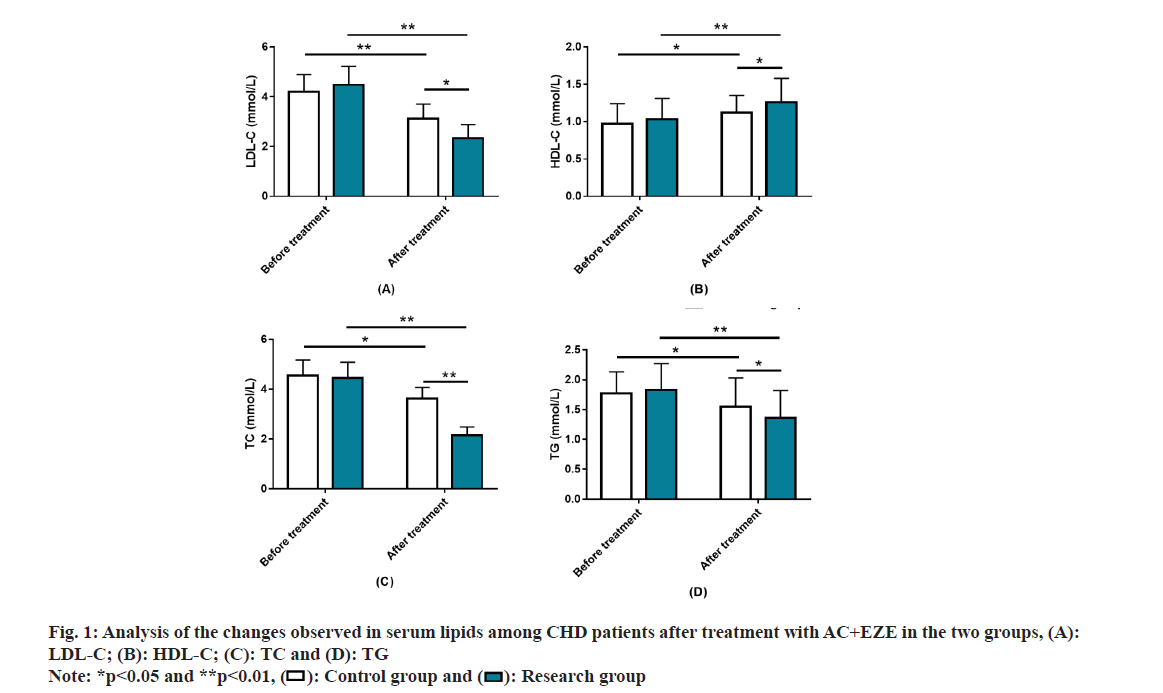
Analysis of the impact of AC+EZE on serum lipids in CHD was studied. LDL-C, HDL-C, TC and TG were measured to assess serum lipid levels in both groups. The above indices were similar between groups prior to treatment (p>0.05). After treatment, LDL-C, TC and TG in both groups decreased while HDL-C increased (p<0.05). Besides, the post-treatment LDL-C, TC, and TG were lower while HDL-C was higher in the research group vs. the other (p<0.05) (fig. 1).
Analysis of the influence of AC+EZE on serum inflammatory markers in CHD was studied. Serum inflammatory markers such as TNF-α, IL-6, and CRP were detected and found to be similar in the research and control groups prior to treatment (p>0.05). All the indexes showed an obvious reduction in both patient cohorts after treatment (p<0.05), particularly in the research group (p<0.05) (fig. 2).
Analysis of the impact of AC+EZE on OS in CHD was evaluated. No significant inter-group differences were found in OS before treatment, as indicated by the pre-treatment MPO, MDA, and SOD levels (p>0.05). After treatment, MPO and MDA decreased and SOD increased, to all the varying degrees (p<0.05). Furthermore, the research group had lower post-treatment MPO and MDA and higher SOD than the control group (p<0.05) (fig. 3).
CHD is a common cardiovascular condition, and its treatment is mainly based on regulating lipid metabolism, mitigating inflammation, and reducing OS[10]. In this study, an evidently higher total effective rate was determined in the research group vs. control group, indicating that the combination of AC and EZE can significantly improve the therapeutic effect of CHD patients. The 95 CHD patients mainly suffered from adverse events such as GIRs, myalgia, loss of appetite, and rash, with an overall incidence 3.56 % less in the research group than in the control group but with no significant inter-group difference, suggesting that AC+EZE would not increase the risk of adverse drug reactions in CHD patients with a favorable safety profile. Foody et al.[11] also demonstrated better efficacy of combined effect of AC and EZE than EZE alone in the regulation of key lipid parameters in elderly CHD patients and equivalent tolerance, similar to our findings. Prasad et al.[12] reported that low-dose AC+EZE had relatively fewer side effects than high-dose atorvastatin, consistent with the results of our study.
Dyslipidemia has been shown to induce atherosclerosis and may progress to CHD and coronary artery disease[13]. Therefore, active regulation and control of dyslipidemia has become an effective means to curb the progression of atherosclerosis and prevent the deterioration of CHD[14]. In our study, the research group showed an evident reduction in LDL-C, TC, and TG after treatment, lower than the baseline and the control group, while HDL-C was markedly elevated, indicating that AC+EZE has a synergistic enhancement effect on blood lipid regulation in CHD patients. This may be attributed to the fact that AC mainly inhibits liver cholesterol production while EZE primarily blocks intestinal cholesterol absorption, playing complementary roles in blood lipid regulation[15].
In the research of Ying et al.[16], EZE can be used to prevent cardiovascular events and plays a positive role in reducing LDL-C and preventing LDL-C from increasing, similar to our research results. Hao et al.[17] also pointed out that EZE+AC can significantly reduce LDL-C and ameliorate LDL-C levels, which supports our research results. In addition, atherosclerosis is an inflammatory disease in essence, and abnormalities in the inflammatory microenvironment can mediate different stages of plaque development and lead to disease deterioration. Specifically, atherosclerosis-induced activation of inflammation induces endothelial cells to secrete adhesion molecules that recruit inflammatory cells to cause early plaque lesions. Smooth muscle cells in plaques will also cooperate with endothelial cells to promote the release of pro-inflammatory mediators, which will lead to the transformation of macrophages into foam cells to further intensify inflammation, thus inducing more immune cells and smooth muscle cells to migrate into plaques and exacerbate disease development.
After treatment, TNF-α, IL-6, and CRP were also evidently lower in the research group vs. the other, suggesting that the abnormal serum inflammatory microenvironment of CHD patients can be effectively controlled under the intervention of AC+EZE. In a mouse CHD model experiment, AC inhibited the activities of Matrix Metalloproteinase (MMP)-2 and MMP-9 and down-regulated the expression of MMP-12, TNF-α and IL-1β, thus reducing the risk of coronary artery stenosis and myocardial infarction, which is similar to our research results[18]. Uzokov et al.[19] also reported the significant inhibitory action of EZE against TNF-α, IL-6, and high-sensitivity C-Reactive Protein (hs-CRP) in patients with CHD and metabolic syndrome, similar to our research results. Besides, the occurrence of OS is usually closely related to changes in metabolic state, lipid peroxidation and other processes, and can mediate the pathogenesis of atherosclerosis[20].
According to our analysis results, the MPO and MDA in the research group reduced markedly after treatment, lower compared with the control group, while the SOD elevated and was higher, indicating that AC+EZE has a good inhibitory effect on OS in CHD patients. The anti-inflammatory and OSrelieving effects of EZE may be related to its regulation of Adenosine 5’-Monophosphate (AMP)- activated Protein Kinase (AMPK)/Nuclear factor erythroid 2-related factor 2 (Nrf2)/Thioredoxin Interacting-Protein (TXNIP) pathway[21].
Taken together, AC+EZE play an important role to provide synergistic effect on improving the curative effect of CHD without increasing adverse drug reactions. In addition, the combined regimen can inhibit dyslipidemia and significantly alleviate serum inflammatory markers and OS, providing an optimized treatment for CHD.
Funding:
This study was supported by Natural Science Foundation of Heilongjiang Province of China (No: LH2020H032).
Author’s contributions:
Jiangbo Yu and Dongli Zhu equally contributed to this work and they are the co-first authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhihua Y, Yingzuo B, Zuo Z, Jinzhe L, Ruijuan Z, Xiaoyu R, et al. A brief discussion on the impact on the quality of life of patients with stable coronary heart disease. J Tianjin Univ Tradit Chin Med 2023;42(2):162-65.
- Xie Q, Li JF. Analysis of changes in the structure of hospitalization expenses for coronary heart disease patients in a tertiary hospital in Nanjing. Chin Med Rec 2023;11:58-60.
- Harman JL, Sayers J, Chapman C, Pellet-Many C. Emerging roles for neuropilin-2 in cardiovascular disease. Int J Mol Sci 2020;21(14):1-18.
[Crossref] [Google Scholar] [PubMed]
- McNeely C, Markwell S, Vassileva CM. Readmission after inpatient percutaneous coronary intervention in the Medicare population from 2000 to 2012. Am Heart J 2016;179:195-203.
[Crossref] [Google Scholar] [PubMed]
- Zhu CH. Exploration of the impact of home care intervention on preventing and reducing the incidence of coronary heart disease recurrence and complications. China Sci Technol J 2022;1:3-9.
- Fu JL, Dong M, Ding L The application effect of Continuity Fox on WeChat platform in secondary prevention after PCI in patients with coronary heart disease. J Aerosp Med 2023;34(5):635-8.
- Mali Y. Effect of tongxinluo and atorvastatin on Lipid metabolism and cardiac function in patients with coronary heart disease. Mod Diag Treat 2017;28(21):1-5.
- Zhang QX. Clinical observation of combined trimetazidine and atorvastatin calcium in 104 coronary heart disease patients with angina pectoris and dyslipidemia. J Front Med 2022;12(29):1-3.
- Gao X. The lipid-lowering efficacy of atorvastatin and ezetimibe in combination for patients with unstable angina. Dalian Med Univ 2014;2:205.
- Wang Y, Liu X, Shi H, Yu Y, Yu Y, Li M, et al. NLRP3 inflammasome, an immune‐inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med 2020;10(1):91-106.
[Crossref] [Google Scholar] [PubMed]
- Foody JM, Brown WV, Zieve F, Adewale AJ, Flaim D, Lowe RS, et al. Safety and efficacy of ezetimibe/simvastatin combination vs. atorvastatin alone in adults ≥65 y of age with hypercholesterolemia and with or at moderately high/high risk for coronary heart disease (the VYTELD study). Am J Cardiol 2010;106(9):1255-63.
[Crossref] [Google Scholar] [PubMed]
- Prasad A, Datta PP, Roy R, Pattanayak C, Panda P. Comparative study of ezetimibe and atorvastatin alone and in combination on lipid profile in rats. Mater Sociomed 2013;25(3):192.
[Crossref] [Google Scholar] [PubMed]
- Budoff MJ, Young R, Burke G, Jeffrey CJ, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with Atherosclerotic Cardiovascular Disease (ASCVD) events: The Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J 2018;39(25):2401-8.
[Crossref] [Google Scholar] [PubMed]
- Hassan A, Din AU, Zhu Y, Zhang K, Li T, Wang Y, et al. Updates in understanding the hypocholesterolemia effect of probiotics on atherosclerosis. Appl Microbiol Biotechnol 2019;103:5993-6006.
[Crossref] [Google Scholar] [PubMed]
- Sarigianni M, Katsiki N, Mikhailidis DP. Ezetimibe in diabetes: More than cholesterol lowering? Curr Med Res Opin 2010;26(10):2517-20.
[Crossref] [Google Scholar] [PubMed]
- Ying Z. The efficacy of trimetazidine combined with ezetimibe in patients with coronary heart disease and its impact on LDL-C, TC, and TG levels. Med Innov China 2022;19(2):61-5.
- Hao T. Effect of atorvastatin calcium combined with new ezetimibe on blood lipid management in patients with coronary heart disease. China Health Care Nutr 2018;28(25):394-98.
- Roth L, Rombouts M, Schrijvers DM, Martinet W, de Meyer GR. Cholesterol-independent effects of atorvastatin prevent cardiovascular morbidity and mortality in a mouse model of atherosclerotic plaque rupture. Vascul Pharmacol 2016;80:50-8.
[Crossref] [Google Scholar] [PubMed]
- Uzokov J, Alyavi A, Alyavi B, Azizov S. Influence of combined therapy on inflammatory state and pro-inflammatory cytokines in patients with coronary artery disease and metabolic syndrome. Eur Cardiol 2020;15.
[Crossref] [Google Scholar] [PubMed]
- Pervaiz S. Redox dichotomy in cell fate decision: Evasive mechanism or Achilles heel? Antioxid Redox Signal 2018;29(13):1191-5.
[Crossref] [Google Scholar] [PubMed]
- Yu J, Wang WN, Matei N, Li X, Pang JW, Mo J, et al. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid Med Cell Longev 2020:1-14.
[Crossref] [Google Scholar] [PubMed]
 ): Control group and (
): Control group and ( ): Research group
): Research group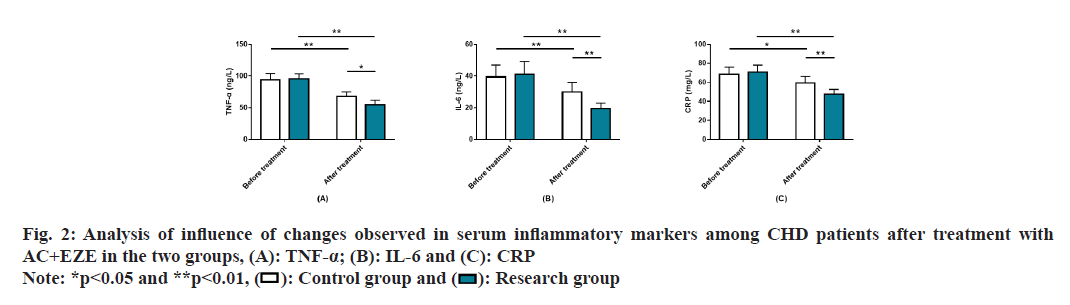
 ): Control group and (
): Control group and ( ): Research group
): Research group 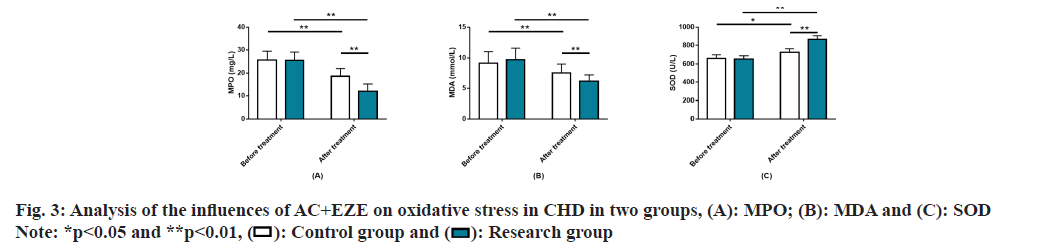
 ): Control group and (
): Control group and (  ): Research group
): Research group 



