- *Corresponding Author:
- H. Zhou1
Department of Gynecology, Zhuzhou Hospital Affiliated to Xiangya School of Medicine, Central South University, Zhuzhou, Hunan 412000, P. R. China
E-mail: zhouhong2025@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “152-158” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study is to retrospectively analyze the therapeutic effects of low-risk invasive mole treatment by methotrexate and summarize the high-risk factors which affect the chemotherapy outcome. Collect the clinical data of low-risk invasive mole treatment by methotrexate and analyze the relationship of human chorionic gonadotropin levels and largest tumor sizes with chemotherapy cycle and toxicity in Zhuzhou Central Hospital from 2015 to 2020. In this study, 42 patients of low-risk invasive mole treatment by methotrexate were enrolled in Zhuzhou Central Hospital from 2015 to 2020. All patients are treated by single-agent methotrexate, the remission rates are 80.95 %, the resistance rates are 11.91 % and the relapse rates are 7.14 %. After change of chemotherapy regimen, the cure rates were 100 %. Age, antecedent pregnancy, International Federation of Gynecology and Obstetrics staging and World Health Organization scoring has no significant difference (p=0.785, p=0.412, p= 0.135 and p=0.135, respectively). However, during the pre-treatment, human chorionic gonadotropin levels and largest tumor sizes were significant factors in the study (p=0.017 and p<0.0001). In addition, serum human chorionic gonadotropin levels and largest tumor size were significant risk factors which show impact on the chemotherapy cycles (p=0.023 and p=0.001). In this study, 10 patients have grade 2, 3 patients have grade 3 and 3 patients have grade 4. After careful study, there was no change in chemotherapy plan or death due to chemotherapy toxicity. In this retrospective study, we explained the single-agent methotrexate for the treatment of lowrisk invasive mole, the remission and resistance, and the relapse and chemotherapy toxicity was correlated with serum human chorionic gonadotropin levels and largest tumor size.
Keywords
Methotrexate, gestational trophoblastic neoplasia, low-risk invasive mole, chemotherapy toxicity
Gestational Trophoblastic Disease (GTD) is a group of benign and malignant tumors originating from uterine and placental tissue[1]. The incidence rate of GTD varies among different regions of the world and the incidence rate of Asian population is higher than that of Europe and North America[2]. According to the report of United States, about 1 time per 1000 pregnancies were diagnosed with GTD[3]. Hydatidiform mole is the most common type of GTD, also known as hydatidiform mole pregnancy, which is considered to be a benign precancerous lesion. Gestational Trophoblastic Neoplasia (GTN) is the general type among malignant types of GTD, including invasive mole, choriocarcinoma, Placental Site Trophoblastic Tumour (PSTT) and Epithelioid Trophoblastic Tumor (ETT)[4]. Hydatidiform mole accounts for about 80 % of all GTD, invasive mole accounts for 15 %, choriocarcinoma and other rare types account for 5 %[4]. GTN is a curable disease with a cure rate of nearly 100 % and treatment can usually preserve reproductive function[5]. According to International Federation of Gynecology and Obstetrics, 2000 (FIGO, 2000), GTN is divided into high risk and low risk. The staging scores system ≤6 belongs to low-risk and ≥7 belongs to high-risk GTN.
The treatment rate of low-risk GTN with single chemotherapy regimen is as high as 70 %-90 %[6-8]. Methotrexate (MTX) or Actinomycin-D (ActD) are recommended single-agent chemotherapy regimen[1]. At present, there are also many literature reports that 5-Fluorouracil (5-FU) is very effective as a single agent in the treatment of low-risk GTN. However, the use of 5-FU is mainly concentrated in Asian countries[9]. High-risk GTN treatment was recommended with the combination chemotherapy, Etoposide, MTX, ActD, Cyclophosphamide, Vincristine (EMA-CO), which is a common combination chemotherapy regimen[1].
Invasive mole is the most common in GTN, approximately 15 % occurred in complete hydatidiform mole[4]. It can invade the myometrium which can even metastasize to lungs, vagina, liver and brain. Low-risk invasive mole cure rate can reach 100 % and high-risk invasive mole cure rate can be 94 %, even if metastasis occurs[10]. However, in the low-risk GTN, there are still a small number of patients, who can show poor prognosis or serious complications. In our study, the retrospective analysis was done by observing the clinical characteristics and prognostic complications which occur during the low-risk invasive mole treatment by single-agent MTX in Zhuzhou Central Hospital from 2015 to 2020. We performed this study, in order to summarize the factors which impact the therapeutic effect of low-risk invasive mole treatment by single-agent MTX.
Materials and Methods
Subjects:
This is a single-center retrospective study in which, we have enrolled 42 patients who were diagnosed with invasive mole by the Pathology department in Zhuzhou Central Hospital from 2015 to 2020. According to World Health Organization (WHO) scoring system, all patients belong to low-risk invasive mole (Table 1).
| WHO risk factor scoring with FIGO staging | Stage 0 | Stage 1 | Stage 2 | Stage 4 |
|---|---|---|---|---|
| Age | <40 | >40 | - | - |
| Antecedent pregnancy | Mole | Abortion | Term | |
| Intervals from index pregnancy, months | <4 | 4-6 | 7-12 | >12 |
| Pretreatment hCG mIU/ml | <103 | >103-104 | >104-105 | >105 |
| Largest tumor size including uterus, cm | - | 3-4 | =5 | - |
| Size of metastases including uterus | Lung | Spleen, kidney | Gastrointestinal tract | Brain and liver |
| Number of metastases identified | - | 1-4 | 5-8 | >8 |
| Previous failed chemotherapy | - | - | Single drug | Two or more drugs |
Note: To stage and allot a risk factor score, a patient’s diagnosis is allocated to a stage as represented by a Roman numerals I, II, III, or IV. This is then separated by a colon from the sum of all the actual risk factor scores expressed in Arabic numerals. Example Stage II: 4, Stage IV: 9. This stage and score will be allotted for each patient
Table 1: WHO Scoring System Based on Prognostic Factors
Methods:
Collect the clinical data of low-risk invasive mole treatment by MTX and analyze the relationship of human Chorionic Gonadotropin (hCG) levels and largest tumor sizes and with chemotherapy cycle and toxicity, in Zhuzhou Central Hospital from 2015 to 2020. We have recorded hCG levels and largest tumor size, before and after each chemotherapy. We have also examined blood cell count and liver and renal function before each course of chemotherapy. Check X-ray of the chest was taken before first chemotherapy which should exclude metastasis. This study was approved by the Ethics Committee of Zhuzhou Central Hospital, Ethical approval No: ZZCHEC2022045-01.
Treatment:
All patients were treated with single-agent MTX, 50 mg/m2 Intramuscularly (IM) weekly. Then serum hCG levels were detected after each chemotherapy cycle, until it is normal and then as per the recommendation of FIGO and for consolidation, at least 2 chemotherapy cycles were performed. Remission is considered as a normal serum hCG levels for 3 consecutive weeks. Resistance is considered as during treatment, the hCG showed plateau or rising levels. In this study, relapse is considered as the hCG rises again after 3 w from normal levels, excluding pregnancy. Chemotherapy complication was screened according to Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0)[11].
Follow-up:
The first follow-up was done after 3 mo of discharge and then 6 mo to 1 y, and then 1 y until 2 y. Generally, pregnancy can be achieved after ≥12 mo after chemotherapy.
Data collection:
Data was collected which included stages, WHO prognostic risk factors and scores, chemotherapy regimen, number of chemotherapy cycles, chemotherapy toxicity, time from resistance and relapse.
Statistical analysis:
The data was analyzed using Statistical Package for Social Sciences (SPSS) 21.0 statistical software and data were expressed as mean±Standard Deviation (SD). The comparison between two groups was performed by t-test, between multiple groups was performed by one-way Analysis of Variance (ANOVA), and the Least Significant Difference (LSD) t-test was performed for pairwise comparison between groups.
Results and Discussion
Patient’s demographic and clinical characteristics were shown in Table 2. We medically recorded 42 patients who were diagnosed with low-risk invasive mole, of which 29 patients (69.04 %) are with the age<40 y and 13 patients (30.96 %) are with the age≥40 y. According to FIGO, 2000 classification regarding stages, 23 cases and 19 cases belong to I and II stages, respectively. Meanwhile, according to the WHO scoring system, 28 patients (66.67 %) and 14 patients (33.33 %) belongs to 1-4 and 5-6 scoring criteria, respectively. Among 42 patients, 39 patients (80.95 %) are under complete remission and 5 patients (11.91%) developed drug resistance and 3 patients (7.14 %) show relapse (Table 2).
| Features | N (%) |
|---|---|
| Age | |
| <40 | 29, (69.04 %) |
| ≥40 | 13, (30.96 %) |
| Antecedent pregnancy | |
| Hydatidiform mole | 0 |
| Abortion | 32, (76.19) |
| Term | 10, (23.81) |
| Largest tumors size | |
| <3 cm | 26, (61.90) |
| 3-5 cm | 9, (21.43) |
| ≥5 cm | 7, (16.67) |
| FIGO staging | |
| I | 23, (54.76) |
| II | 19, (45.24) |
| III | 0 |
| IV | 0 |
| WHO scores | |
| 1-4 | 28, (66.67) |
| 5-6 | 14, (33.33) |
| Pretreatment hCG (IU/l) | |
| <103 | 14, (33.33) |
| 103-104 | 15, (35.72) |
| >104 | 13, (30.95) |
| Chemotherapy effect | |
| Remission | 34, (80.95) |
| Resistance1 | 5, (11.91) |
| Relapse2 | 3, (7.14) |
| Chemotherapy courses (cycles) | |
| ≤3 | 19, (45.24) |
| 4-7 | 15, (35.72) |
| ≥8 | 8, (19.04) |
| Chemotherapy toxicity | |
| Grade 2 | 10, (23.81) |
| Grade 3 | 3, (7.14) |
| Grade 4 | 3, (7.14) |
Note: Resistance1: 2 patients changed chemotherapy regimen to Act-D and 3 patients changed chemotherapy regimen to EMA-CO. Relapse2: 3 patients of relapse who were relieved after four courses of chemotherapy by EMA-CO regimen
Table 2: Patient Demographic and Clinical Characteristics
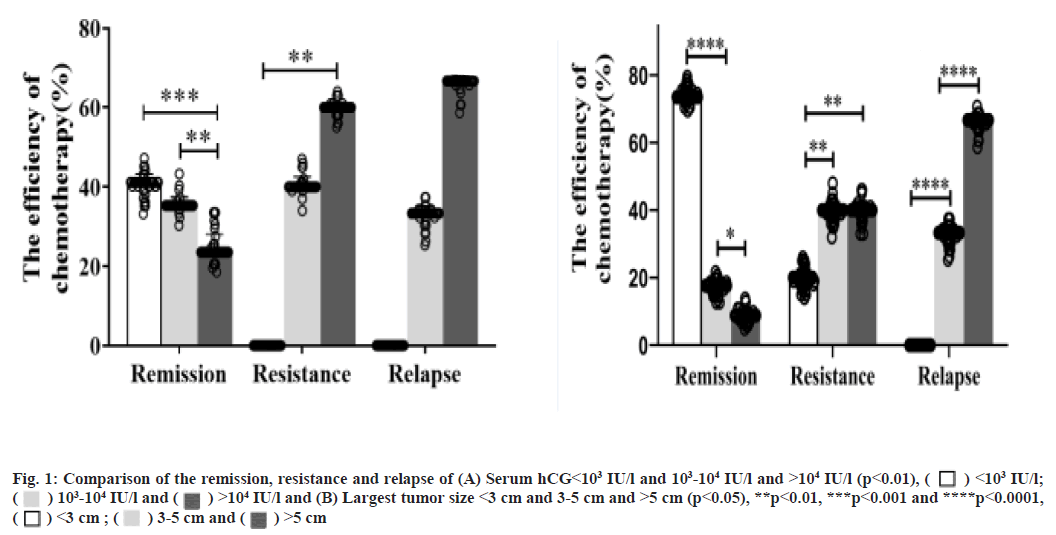
The factors that treat low-risk invasive mole by single-agent MTX was shown here. 42 patients were analyzed and the factors which treat low-risk invasive mole by single-agent MTX were shown in Table 3. As shown in Table 3, for the largest tumors size <3 cm and between 3-5 cm and >5 cm, the remission rates were 96.15 % (25/26), 66.67 % (6/9), 42.86 % (3/7), respectively. For the tumor size <3 cm, 1 patient develop resistance and tumor size between 3-5 cm, 2 patients develop resistance and 1 patient show relapse and tumor size >5 cm, 2 patients develop resistance and 2 patients show relapse. Age, antecedent pregnancy, FIGO stage and WHO scoring show no significant difference (p=0.785, p=0.412, p=0.135 and p=0.135, respectively). However, pre-treatment hCG levels and largest tumor size were significant factors (p=0.017 and p<0.001, respectively). Compare the remission rate and serum hCG<103 International Units (IU)/l which was significantly higher than 103-104 IU/l and >104 IU/l (p<0.001, fig. 1A). The largest tumor size <3 cm was significantly higher than 3-5 cm and >5 cm (p<0.001, fig. 1B).
| Contents | Chemotherapy effect | ||||
|---|---|---|---|---|---|
| Remission | Resistance | Relapse | p | ||
| Age | <40 | 26 | 2 | 1 | 0.785 |
| ≥40 | 8 | 3 | 2 | ||
| Antecedent pregnancy | Abortion | 29 | 2 | 1 | 0.412 |
| Term | 5 | 3 | 2 | ||
| FIGO Stage | I | 22 | 1 | 0 | 0.135 |
| II | 12 | 4 | 3 | ||
| WHO scoring | 1-4 | 22 | 1 | 0 | 0.135 |
| 5-6 | 12 | 4 | 3 | ||
| Pre-treatment hCG levels | <103 IU/l | 14 | 0 | 0 | 0.017 |
| 103-104 IU/l | 12 | 2 | 1 | ||
| >104 IU/l | 8 | 3 | 2 | ||
| Largest tumor size | <3 cm | 25 | 1 | 0 | <0.0001 |
| 3-5 cm | 6 | 2 | 2 | ||
| >5 cm | 3 | 2 | 1 | ||
Note: Serum hCG levels <103 IU/l, all patients achieved remission. Serum hCG levels between 103-104 IU/l, 80 % (12/15) patients achieved remission, drugs-resistance is seen in 2 patients and relapse in 1 patient. Serum hCG levels >104 IU/l, 61.54 % (8/13) patients achieved remission and resistance is seen in 3 patients and relapse in 2 patients
Table 3: The Factors That Treat Low-Risk Invasive Mole by Single-Agent MTX
Serum hCG levels and largest tumor size affect chemotherapy cycles and toxicity. There are 20 patients with chemotherapy cycles ≤4, 15 patients with chemotherapy cycles between 5-7 and 7 patients with chemotherapy cycles ≥8. When hCG<103 IU/l, the chemotherapy cycles were significantly shorter than 103-104 IU/l and >104 IU/l (85.71 % vs. 36.84 % vs. 0, p<0.0001, Table 4 and fig. 2A). Meanwhile, when tumor size<3 cm chemotherapy cycles were significantly shorter than 3-5 cm and >5 cm (67.9 % vs. 13.33 % vs. 0, p<0.0001, Table 4 and fig. 2B).
| Contents | Serum hCG levels (IU/l) | |||||
|---|---|---|---|---|---|---|
| <103 | 103-104 | >104 | <3 | 3-5 | >5 | |
| Chemotherapy cycles | ||||||
| ≤4 | 12 (85.71 %) | 7 (46.67 %) | 0 | 20 (76.92 %) | 2 (13.33 %) | 0 |
| 5-7 | 2 (14.29 %) | 5 (33.33 %) | 8 (61.54 %) | 6 (14.29 %) | 9 (60 %) | 9 (69.23 %) |
| ≥8 | 0 | 3 (20 %) | 5 (38.46 %) | 0 | 4 (26.67 %) | 4 (30.77 %) |
| p | 12 vs. 2 vs. 0 | 7 vs. 3 | 0 vs. 8 vs. 5 | 20 vs. 6 vs. 0 | 2 vs. 9 vs. 4 | 0 vs. 9 vs. 4 |
| p<0.0001 | p<0.001 | p<0.001 | p<0.0001 | p<0.0001 | p<0.0001 | |
Table 4: Serum hCG Levels and Largest Tumor Size Affect Chemotherapy Cycles
10 patients have grade 2, 3 patients have grade 3 and 3 patients have grade 4 chemotherapy toxicity (Table 5). After careful care, there was no change in chemotherapy plan or death due to chemotherapy toxicity.
| Toxicity | Chemotherapy cycles | ||
|---|---|---|---|
| ≤4 | 5-7 | ≥8 | |
| Grade 2 | 6 | 4 | 0 |
| Grade 3 | 0 | 1 | 2 |
| Grade 4 | 0 | 1 | 2 |
Note: 10 patients have grade 2, 3 patients have grade 3 and 3 patients have grade 4, chemotherapy toxicity
Table 5: Relationship between Chemotherapy Cycle and Toxicity
According to the recommendations of 2021 National Comprehensive Cancer Network (NCCN) and FIGO 2000 of GTN, MTX is the most common chemotherapeutic drug used for the treatment of low-risk invasive mole. However, some patients still need to change chemotherapy drugs due to recurrence, resistance or severe toxicity. We have analyzed the potential factors that may affect resistance or relapse or severe toxicity among which 42 patients were diagnosed with low-risk invasive mole by MTX treatment in Zhuzhou Central Hospital from 2015 to 2020.
In our study, the overall remission rates after primary single-agent MTX regimen was 80.95 % (34/42). In the same time, we found that serum hCG and largest tumor size are independent factors that affect the therapeutic effect and chemotherapy toxicity. When serum hCG<103 and largest tumor size <3 cm, the therapeutic effect was the best and the chemotherapy cycle is the shortest and the toxicity is the least. HCG>105 IU/l or tumor size>5 cm, the resistance and relapse and toxicity were significantly higher than hCG<103 IU/l or tumor size <3 cm. Phianpiset et al.[12], analyzed 113 patients of low-risk GTN and they found that serum hCG≥15 000 IU/l which is an independent factor to predict the failure of treatment by Methotrexate-Folinic Acid (MTX-FA). A study analyzed 365 patients of low-risk GTN, they have considered hCG>300 IU/l increased the failure risk which is an important factor for the treatment by second-line Act-D[13]. Soper et al.[14] studied 51 patients with 5 d MTX treatment of low-risk GTN, they found that drug resistance was related to the level of hCG levels (>10 000 mIU/ml). In addition, some reports show that the invasive mole tuner size is too large resulting in uterine rupture. Wu et al.[15], in a case report of invasive mole stated that, the tumor size of about 12 cm, results in uterine rupture. Obviously, these literature reports confirm hCG levels and largest tumor size has been used as an important indicator of the efficacy and prognosis of GTN.
Although, FIGO scores 0-6 belongs to low-risk GTN, approximately 30 % of patients with low-risk GTN (FIGO scores 5-6) have responded to these regimens, while reconsidering in scoring and staging system would be effective in recognizing 70 % of resistant patients of this group[3]. In 2011, in a study comparing MTX and Act-D treatment of GTN, they found that initial dose of 30 mg/m2 MTX without escalation has the response rate of 53 %. Finally, they concluded that MTX and Act-D regimen is less effective in low-risk GTN, especially with the scores of 5-6[16]. In our study, the WHO scores were found to be 5-6 for low-risk invasive mole and the overall response rate is 50 %. Our results are consistent with the literature reports. However, our data displayed according to the WHO scoring criteria, has no risk factor in MTX invasive mole treatment. The data we collected may be related only to the less patients.
In addition to achieve long-term cure, minimizing toxicity is an important factor in evaluating the treatment. Drug toxicity is related to the course of chemotherapy. Some studies use Folic Acid (FA) to reduce the MTX toxicity. Some studies suggest that 11 %-31 % patients of low-risk GTN treatment by MTX, requires change in chemotherapy regimen because of intolerable toxicity[17,18]. In our study, there are 6 patients with grade 3-4 toxicity, who are proportion to the total patient’s rate of 14.29 % (6/42). These patients mainly focused on chemotherapy cycles≥8. At the same time, the occurrence of grade 3-4 chemotherapy toxicity was associated with serum hCG levels and largest tumor size. The serum hCG levels>103 IU/l and the largest tumor size >3 cm directly affects the course of chemotherapy and chemotherapy toxicity.
In conclusion, although our study is a small sample retrospective study, from our data, it is clear that, when the levels of hCG<103 IU/l and the largest tumor size<3 cm was present, single-agent MTX treatment is the best for low-risk invasive mole. However, when hCG>104 IU/l or the largest tumor size>5 cm or in low-risk GTN of score 5-6, multidrug chemotherapy should be considered.
Author’s contributions:
Genlin Liu and Le Li contributed equally to this work. Genlin Liu and Hong Zhou: Conceptualization, writing-original draft, methodology, formal analysis. Ting Sun: Supervision, validation, data curation. Xiaoyan He: Investigation, software, visualization. Le Li: Funding acquisition, project administration, resources, writing-review and editing.
Conflict of interests:
The authors declare no potential conflicts of interest.
References
- Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, et al. Gestational trophoblastic neoplasia, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17(11):1374-91.
[Crossref] [Google Scholar] [PubMed]
- Lurain JR. Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease and management of hydatidiform mole. Am J Obstet Gynecol 2010;203(6):531-9.
[Crossref] [Google Scholar] [PubMed]
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet 2010;376(9742):717-29.
[Crossref] [Google Scholar] [PubMed]
- Brown J, Naumann RW, Seckl MJ, Schink J. 15 years of progress in gestational trophoblastic disease: Scoring, standardization, and salvage. Gynecol Oncol 2017;144(1):200-7.
[Crossref] [Google Scholar] [PubMed]
- Lurain JR. Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol 2011;204(1):11-8.
[Crossref] [Google Scholar] [PubMed]
- Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynecol Obstet 2021;155(1):86-93.
[Crossref] [Google Scholar] [PubMed]
- Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecol Obstet 2018;143(2):79-85.
[Crossref] [Google Scholar] [PubMed]
- Goldstein DP, Berkowitz RS, Horowitz NS. Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anticancer Ther 2015;15(11):1293-304.
[Crossref] [Google Scholar] [PubMed]
- Peng M, Ding Y, Yu L, Deng Y, Lai W, Hu Y, et al. Tegafur substitution for 5-Fu in combination with actinomycin D to treat gestational trophoblastic neoplasm. PLoS One 2015;10(11):e0143531.
[Crossref] [Google Scholar] [PubMed]
- Alifrangis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, et al. EMA/CO for high-risk gestational trophoblastic neoplasia: Good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol 2013;31(2):280-6.
[Crossref] [Google Scholar] [PubMed]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, 2017; 2021.
- Phianpiset R, Ruengkhachorn I, Kuljarusnont S, Jareemit N, Udompunturak S. Predictive factors associated with resistance to initial methotrexate treatment in women with low‐risk gestational trophoblastic neoplasia. Asia Pac J Clin Oncol 2022.
[Crossref] [Google Scholar] [PubMed]
- Maestá I, Nitecki R, Desmarais CC, Horowitz NS, Goldstein DP, Elias KM, et al. Effectiveness and toxicity of second-line actinomycin D in patients with methotrexate-resistant postmolar low-risk gestational trophoblastic neoplasia. Gynecol Oncol 2020;157(2):372-8.
[Crossref] [Google Scholar] [PubMed]
- Soper JT. Gestational trophoblastic disease: Current evaluation and management. Obstet Gynecol 2021;137(2):355.
[Crossref] [Google Scholar] [PubMed]
- Wu A, Zhu Q, Tan C, Chen L, Tao Y. Invasive mole resulting in uterine rupture: A case report. Front Surg 2021;8:798640.
[Crossref] [Google scholar] [PubMed]
- Osborne RJ, Filiaci V, Schink JC, Mannel RS, Secord AA, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: A gynecologic oncology group study. J Clin Oncol 2011;29(7):825-31.
[Crossref] [Google Scholar] [PubMed]
- McNeish IA, Strickland S, Holden L, Rustin GJ, Foskett M, Seckl MJ, et al. Low-risk persistent gestational trophoblastic disease: Outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol 2002;20(7):1838-44.
[Crossref] [Google Scholar] [PubMed]
- Li J, Li S, Yu H, Wang J, Xu C, Lu X. The efficacy and safety of first-line single-agent chemotherapy regimens in low-risk gestational trophoblastic neoplasia: A network meta-analysis. Gynecol Oncol 2018;148(2):247-53.
[Crossref] [Google Scholar] [PubMed]
 <103 IU/l;
<103 IU/l;  103-104 IU/l and
103-104 IU/l and  >104 IU/l and (B) Largest tumor size <3 cm and 3-5 cm and >5 cm (p<0.05), **p<0.01, ***p<0.001 and ****p<0.0001,
>104 IU/l and (B) Largest tumor size <3 cm and 3-5 cm and >5 cm (p<0.05), **p<0.01, ***p<0.001 and ****p<0.0001,  >5 cm
>5 cm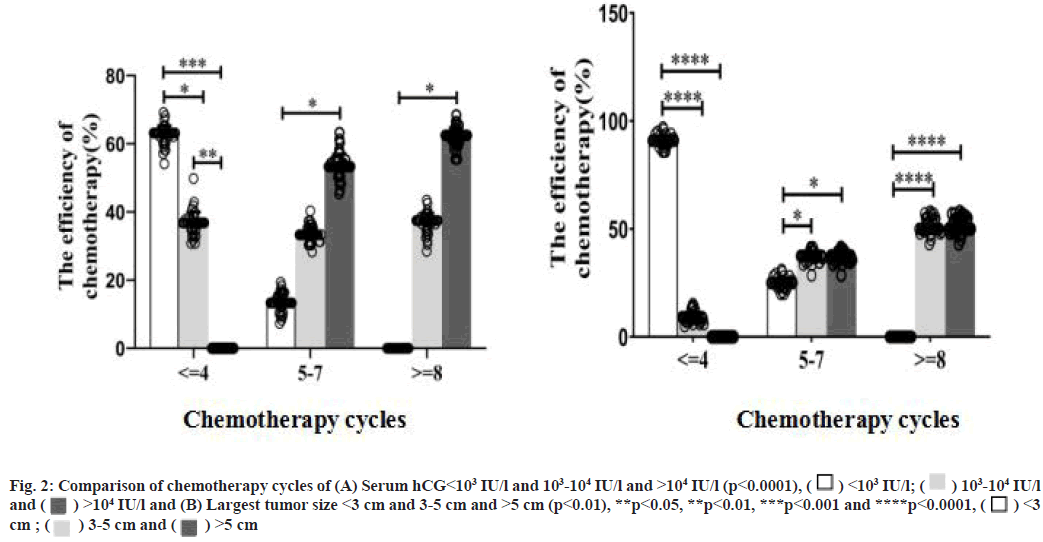
 103-104 IU/l and
103-104 IU/l and  >104 IU/l and (B) Largest tumor size <3 cm and 3-5 cm and >5 cm (p<0.01), **p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001,
>104 IU/l and (B) Largest tumor size <3 cm and 3-5 cm and >5 cm (p<0.01), **p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001,  <3 cm ;
<3 cm ; >5 cm
>5 cm



