- *Corresponding Author:
- Yanhuan Zhang
Administrative Department, Guoyang County People’s Hospital, Bozhou, Anhui Province 233600, China
E-mail: 175822174@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “75-83” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze the clinical efficacy and assess the safety of karelizumab combined with apatinib in the treatment of intermediate and advanced hepatocellular carcinoma. Forty-four patients diagnosed with intermediate and advanced hepatic malignant tumors in our hospital were retrospectively collected from January 2019 to June 2020, and the patients were divided into karelizumab combined with abatinib group A (22 patients) and abatacept group B (22 patients) according to the different treatment methods. Baseline data, clinical outcomes after 1 mo, 3 mo and 6 mo of follow-up were compared between the two groups. The follow-up period was up to December 31, 2022 and patients were counted for progression-free overall survival, and progression-free survival. Treatment-related adverse events were assessed using the common terminology criteria for adverse events. Risk factors affecting progression-free survival were analyzed using univariate and multivariate analysis with Cox risk regression model. Treatment-related adverse events were assessed using the common terminology criteria for adverse events. The best outcome of each patient was recorded; complete response, partial response, stable disease and progressive disease at the best outcome were 9 (20.45), 20 (45.45 %), 7 (15.91 %) and 8 (18.18 %), respectively. The objective remission rate of karelizumab combined with apatinib in the treatment of intermediate and advanced hepatocellular carcinoma is high and the safety is good, which lays the foundation for the subsequent clinical trials to be carried out.
Keywords
Hepatocellular carcinoma, mortality, karelizumab, apatinib, tumor
Primary Liver Cancer (PHC) is a common malignant tumor worldwide, and its incidence has shown a significant upward trend in recent years, while the high mortality rate of this disease has seriously affected the quality of survival and health status of patients[1,2]. Because the early clinical manifestations of primary Hepatocellular Carcinoma (HCC) are often not easy to be detected, resulting in the majority of cases have already progressed to the middle and late stages when diagnosed, and the best time for surgical treatment is missed[3]. As the treatment of choice for advanced HCC, Transcatheter Arterial Chemoembolization (TACE) has achieved significant results in prolonging patient’s survival; nevertheless, this treatment still faces certain side effects as well as a higher risk of tumor recurrence and metastasis[4].
Immune Checkpoint Inhibitors (ICIs) belong to a class of innovative monoclonal antibody drugs designed to promote immune responses to tumors by resisting inhibitory immune receptors[5]. Among all checkpoint molecules, antibodies against Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4), Programmed Cell Death Protein 1 (PD-1), and Programmed Death Receptor Ligand 1 (PD-L1) are the most effective. PD-L1 have been most intensively studied[6], and these molecules have important clinical value in human cancer therapy. Tumor cells achieve immune escape by binding to PD-1 on T cells through PDL1, while the application of PD-1/PD-L1 inhibitors can block this pathway and enhance the tumor recognition and clearance function of T cells[7]. Karelizumab belongs to a humanized anti-PD-1 monoclonal antibody with high affinity developed in China, marking a breakthrough in the field of PD-1 inhibitors, which was approved as a secondline treatment option for intermediate and advanced HCC in 2020. However, the objective remission rate of ICIs alone in intermediate- and advanced-stage HCC is about 20 %, pointing out the need to optimize treatment strategies[8]. By modulating the tumor microenvironment and T-cell activity, anti-angiogenic drugs may enhance the effect of ICIs, providing a theoretical basis for combination therapy[9]. In addition, the combination of apatinib and karelizumab, as an innovative therapeutic regimen developed in China, has been approved for the treatment of intermediate and advanced HCC in 2020. The aim of this study was to evaluate the efficacy and safety of this combination regimen in patients with intermediate and advanced primary HCC, and to provide a reference for clinical treatment.
Materials and Methods
Objects of study:
In this study, a retrospective method was adopted to collect the clinical data of 44 patients with intermediate and advanced HCC diagnosed in our hospital during the period from September 2020 to August 2022, with a balanced gender distribution of 22 patients of each gender, aged 49 y-78 y old, with a mean age of (63.8±11.5) y old. According to the child-Pugh grading system, there were 25 patients with grade A (29.07 %) and 61 patients with grade B (70.93 %). According to the Barcelona Staging System for HCC (BCLC), 56 patients (65.12 %) were in stage B and 30 patients (34.88 %) were in stage C. The number of TACE treatments received by the patients ranged from 1 to 4, with a mean number of TACE sessions (2.1±0.4), of which 15 patients (17.44 %) showed resistance to TACE treatment, 7 (8.14 %) were combined with distant metastases, 59 (68.60 %) had tumors with a diameter of >5 cm, 14 (16.28 %) were combined with hypertension, 12 (13.95 %) combined with diabetes mellitus, and 71 (82.56 %) combined with hepatitis B.
Inclusion criteria: Patients with primary HCC confirmed by pathological examination, imaging evaluation, and serologic analysis; classified as grade A or B according to the Child-Pugh criteria; with a Karnofsky Performance Status (KPS) score of >80; with detailed routine clinical information and laboratory test results and patients who had not received, prior to admission, an immunotherapy or targeted therapy.
Exclusion criteria: Patients who had previously used apatinib or other PD-1/PD-L1 inhibitors[10]; patients with other primary tumors or autoimmune liver disease; patients with severe infections; patients with grade 4 or higher toxicities or allergic reactions; patients who were unable to satisfy the requirements for follow-up during treatment, voluntarily gave up the treatment, or were transferred to other hospitals to patients with a history of major bleeding in the last 30 d and patients with hypertension that is difficult to control with medication. The study was approved by the hospital ethics committee and complied with local legal standards in China, and the medical record information was obtained through the electronic medical record system, exempting patients from informed consent.
Medication regimen:
Upon hospitalization, participants will undergo the necessary examinations and be fully informed of the possible toxicities associated with the treatment, and after confirming that there are no contraindications to immunologic and targeted therapies, patients will be required to sign an informed consent form for immunotherapy and biomolecular-targeted therapies. On the 1st d of treatment, patients received a fixed dose of 200 mg of carilizumab, which was reconstituted in 5 ml of sterile water for injection, and then diluted into 100 ml of 5 % dextrose solution or 0.9 % sodium chloride solution, and was administered by intravenous drip, with a controlled drip time of 30 min to 60 min. In addition, from the 1st to 21st d, patients were given a daily oral dose of abatinib mesylate tablets of 250 mg for 21 d. 250 mg for 21 d as a treatment cycle, and at least 2 treatment cycles were performed. For patients with a favorable response, after completion of 9 treatment cycles, maintenance therapy with karelizumab in combination with apatinib may be instituted, and treatment will continue until the patient develops intolerable toxicity, definitive progression of disease, death, or withdrawal of consent. For patients who fail to respond to treatment, the treatment regimen will be changed. If a patient shows disease progression on imaging but has controlled symptoms and is well tolerated with either carilizumab or apatinib, the investigator may decide to continue treatment beyond initial disease progression.
Assessment of clinical efficacy:
Adopting the revised solid tumor efficacy evaluation criteria (mRECIST) of the American Association for the Study of Liver Diseases (AASLD)[11], the efficacy was evaluated by the patient’s imaging data (preferentially evaluated by abdominal-enhanced MR, or abdominal-enhanced CT in the absence of MR). The efficacy evaluation was divided into the following categories; Complete Response (CR), Partial Response (PR), Stable Disease (SD), Progressive Disease (PD), Objective Response Rate (ORR), and the number of patients who had been treated with mRECIST[11]. ORR and Disease Control Rate (DCR) were defined as the proportion of CR+PR cases and DCR as the proportion of CR+PR+SD cases. OS was defined as the time from the start of the first Hepatic Arterial Infusion Chemotherapy (HAIC) treatment to the time of death due to any cause or the cut-off of follow-up, and Progression- Free Survival (PFS) was defined as the time from the start of the first HAIC treatment to the cut-off of follow-up. Overall Survival (OS) was defined as the time from the start of the first HAIC treatment to the first occurrence of disease progression or death from any cause or cutoff of follow-up, and if a patient was lost to follow-up before death, the time of his or her last follow-up was counted as the time of death.
Follow-up visits:
The follow-up process was primarily during the patient’s regular hospitalization, supplemented by outpatient visits and telephone contacts to ensure completeness and accuracy of information. The follow-up period begins on the day the patient begins treatment with carilizumab plus apatinib or apatinib alone and continues until 12 mo after treatment. During this period, the patient’s response to treatment, survival and any associated health changes will be recorded in detail. The last point of follow-up is set as the patient’s disease progression, death from any cause, or until December 31, 2022, whichever comes first. This method of follow-up is designed to comprehensively assess the long-term efficacy and quality of survival of patients after receiving the novel treatment regimen in order to provide an important reference for future clinical decisions.
Drug safety evaluation:
Adverse reactions generated during patient treatment were evaluated using the Common Terminology Criteria for Adverse Events version 5.0 (PRO-CTCAE 5.0) adopted by the National Cancer Institute[12], and if the patient’s adverse reactions were >grade 4, the treatment regimen was discontinued or changed.
Statistical methods:
In this study, data processing was executed using Statistical Package for the Social Sciences (SPSS) 26.0 statistical software. For the measured data obeying normal distribution, the data were presented in the form of mean±standard deviation (x̄±s) were presented, while the comparison of differences between groups was done by one-way Analysis of Variance (ANOVA). Comparisons of count data were performed using the Chi-square (χ2) test or Fisher’s exact test. Survival time analysis was performed by the Kaplan-Meier curve method, and the risk factors potentially affecting PFS were analyzed by Cox proportional risk regression model in a one-way and multifactor analysis. Differences were considered statistically significant when p<0.05.
Results and Discussion
There was no statistically significant difference in the baseline data of age, sex ratio, BCLC stage, Childpugh classification, Eastern Cooperative Oncology Group (ECOG) score, distant metastasis, portal vein cancer embolism, and ascites between the two groups (p>0.05), as shown in Table 1.
| Sports event | Group A (n=22) | Group B (n=22) | χ2/t | p |
|---|---|---|---|---|
| Age | 53.96±10.51 | 55.52±11.46 | 0.462 | 0.652 |
| Sex (male/female) | 18/4 | 15/7 | 1.091 | 0.296 |
| BCLC staging (B/C) | 9/13 | 9/13 | 0.000 | 1 |
| Child-pugh classification (A/B) | 9/13 | 14/8 | 2.277 | 0.131 |
| ECOG score (1 out of 2) | 11/11 | 16/6 | 2.397 | 0.122 |
| Distant metastasis (cases) | 5 | 3 | 0.434 | 0.611 |
| Portal vein thrombosis (cases) | 5 | 5 | 0.000 | 1 |
| Ascites (yes/no) | 4/18 | 8/14 | 1.833 | 0.176 |
| Maximum tumor diameter (cm) | 7.35±2.92 | 6.29±1.98 | 1.43 | 0.16 |
| Albumin (g/l) | 37.28±5.77 | 38.02±6.77 | 0.377 | 0.708 |
| Albuminous aminotransferase (U/l) | 39.92±57.32 | 33.69±21.32 | 0.478 | 0.636 |
| Glutamine aminotransferase (U/l) | 61.93±63.23 | 51.69±29.91 | 0.699 | 0.492 |
| Alpha-fetoprotein (ng/ml) | 955.39±1102.58 | 1406.35±2971.23 | 0.669 | 0.509 |
| History of alcohol consumption (yes/no) | 4/18 | 8/14 | 1.833 | 0.176 |
| Hepatitis B (yes/no) | 4/18 | 7/15 | 0.296 | 1.091 |
| PS score (0 point/1 point) | 7/15 | 9/13 | 0.531 | 0.393 |
| Number of lesions (>3/≤3) | 14/8 | 19/3 | 3.03 | 0.082 |
Table 1: Comparison of patients general information
In this study, the clinical efficacy of 44 patients with moderately advanced HCC who received treatment was recorded, with special attention paid to the changes in the sum of the arterial enhancement diameters obtained by tumor imaging at 1 mo, 3 mo and 6 mo after treatment. The assessment of treatment efficacy was based on Complete Remission (CR), Partial Remission (PR), SD and Disease Progression (PD), and the results were as follows; 9 cases (20.45 %) in CR, 20 cases (45.45 %) in PR, 7 cases (15.91 %) in SD, and 8 cases (18.18 %) in PD. In addition, in terms of the maximum percentage change in the diameter of the target lesion during the period of optimal efficacy, the relevant data and trends are displayed in fig. 1.
Evaluation of efficacy at 1 mo after treatment the difference between ORR and Polymerase Chain Reaction (PCR) of group A and group B was not statistically significant (p>0.05), evaluation of efficacy at 3 mo of treatment; the difference between PCR of group A and group B was not statistically significant (p>0.05), ORR of group A was higher than that of group B, and the difference was statistically significant (p<0.05), evaluation of efficacy at 6 mo of treatment; ORR of group A, PCR were higher than group B, and the difference was statistically significant (p<0.05), as shown in Table 2.
| Efficacy evaluation | Group A (n=22) | Group B (n=22) | ||||
|---|---|---|---|---|---|---|
| January | March | June | January | March | June | |
| CR | 5 | 6 | 4 | 3 | 3 | 1 |
| PR | 10 | 11 | 8 | 8 | 7 | 4 |
| SD | 4 | 3 | 5 | 6 | 5 | 2 |
| PD | 3 | 2 | 5 | 5 | 7 | 15 |
| ORR | 68.18 (15/22) | 77.27 (17/22) | 54.55 (12/22) | 50.50 (11/22) | 45.45 (10/22) | 22.73 (5/22) |
| DCR | 86.36 (19/22) | 90.90 (20/22) | 77.27 (17/22) | 77.27 (17/22) | 54.55 (12/22) | 31.82 (7/22) |
Table 2: Comparison of clinical efficacy between two groups of patients treated for 1 mo, 3 mo and 6 mo
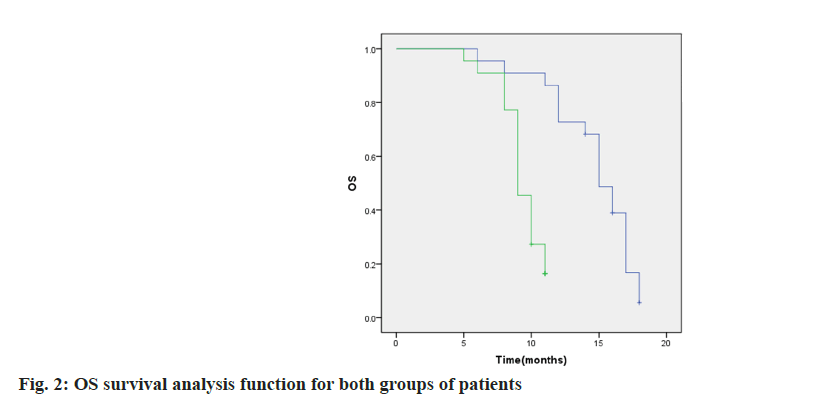
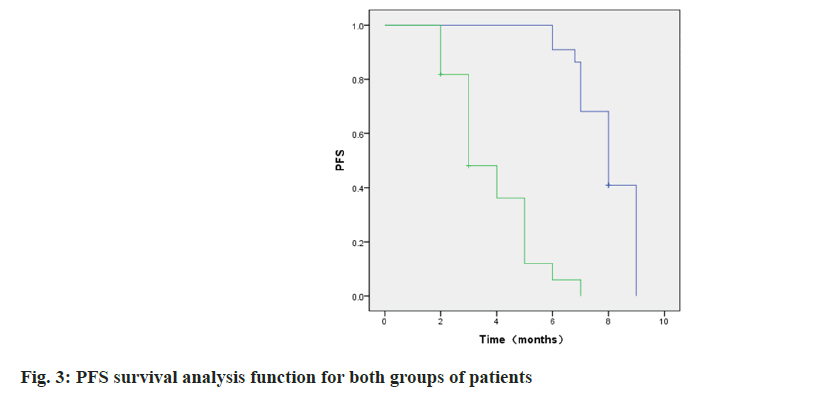
By the end of follow-up 4 patients in group A did not achieve OS and 8 patients in group B. The median OS was 15.00 (95 % CI: 11.66~18.34) in group A and 10.00 (95 % CI: 8.02~11.98) in group B. As shown in fig. 2, p=0.000.1 patient in each of the two groups did not achieve PFS at the end of follow-up, and the group A. The median time to PFS was 8.0 (95 % CI: 6.69~9.31), and the median time to PFS in group B was 5.0 (95 %: 2.70~7.298) mo as shown in fig. 3, p=0.000.
In this study, no treatment-related deaths occurred in either group during the treatment period. Treatment-induced adverse reactions were mainly concentrated in grades 1 and 2, and these symptoms were effectively relieved after receiving appropriate symptomatic treatment without causing harm to the patients. In group A, 12 patients (54.55 %) experienced hypertensive reactions, of which 2 (9.09 %) were grade 3 adverse reactions. While in group B, 14 patients (63.64 %) developed hypertension, of which again 2 (9.09 %) were grade 3 adverse reactions. It is worth noting that the patients who experienced grade 3 adverse reactions had a history of underlying hypertension and had elevated blood pressure after the administration of the drug, but with prompt symptomatic treatment, these patients were able to control their blood pressure below 140/90 mmHg. The difference in the incidence of adverse reactions between the two groups of patients was not statistically significant (p>0.05), which indicates that the combination regimen has better safety and tolerability in patients with intermediate and advanced HCC. The specific occurrences of other adverse reactions were recorded in detail as shown in Table 3.
| Adverse reaction | Combined treatment group (n=22) | Apatinib group (n=22) | ||
|---|---|---|---|---|
| Level 1 to 2 | Level 3 | Level 1 to 2 | Level 3 | |
| Anemic | 3 (13.64) | 0 | 2 (9.09) | 0 |
| Leucopenia | 5 (22.73) | 0 | 5 (22.73) | 0 |
| High blood pressure | 10 (45.45) | 2 (9.09) | 12 (54.55) | 2 (9.09) |
| Have the runs | 6 (27.27) | 0 | 6 (27.27) | 0 |
| Skin capillary hyperplasia | 4 (18.18) | 0 | 1 (4.55) | 0 |
| Gastrointestinal bleeding | 1 (4.55) | 0 | 2 (9.09) | 0 |
| Lose hair or feathers | 5 (22.73) | 0 | 4 (18.18) | 0 |
| Hand-to-foot syndrome | 9 (40.91) | 0 | 8 (36.36) | 0 |
Table 3: Comparison of drug safety between the two groups of patients (number of cases (%))
Univariate analysis showed that there was a correlation between treatment modality, ECOG score, child classification, BCLC stage, and maximum tumor diameter with PFS in patients with intermediate and advanced HCC, and the results of Cox multifactorial analysis showed that treatment modality and child classification were the independent prognostic factors associated with PFS in patients with intermediate and advanced HCC (p<0.05), as shown in Table 4.
| Norm | One-way analysis of variance | Multifactorial analysis | ||
|---|---|---|---|---|
| HR (95 % CI) | p | HR (95 % CI) | p | |
| Age (≤60/>60) | 0.995 (0.601-1.645) | 0.985 | ||
| Sex (male/female) | 0.741 (0.498-1.105) | 0.144 | ||
| Treatment modality (combination/apatinib) | 2.149 (1.410-3.275) | <0.001 | 1.733 (1.097-2.739) | 0.019 |
| History of alcohol consumption (yes/no) | 1.327 (0.882-1.985) | 0.168 | ||
| ECOG score (0 out of 1) | 1.538 (1.011-2.335) | 0.043 | ||
| Child classification (class A/level B) | 1.705 (1.084-2.682) | 0.023 | 1.827 (1.165-2.866) | 0.009 |
| Distant metastases (yes/no) | 1.648 (1.00-2.715) | 0.052 | ||
| Hepatitis B (yes/no) | 1.499 (0.884-2.541) | 0.134 | ||
| PS score (0 points/1 point) | 0.833 (0.529-1.472) | 0.652 | ||
| BCLC staging (B/C) | 1.628 (1.088-2.441) | 0.012 | ||
| Maximum tumor diameter (>5/≤5 cm) | 1.456 (0.817-2.436) | 0.044 | ||
| Number of lesions (>3/≤3) | 0.876 (0.579-1.328) | 0.532 | ||
| Portal vein thrombosis (yes/no) | 1.796 (1.183~2.725) | 0.006 | ||
| Ascites (yes/no) | 0.833 (0.529-1.473) | 0.633 | ||
| Albumin (>35 g/l/≤35 g/l) | 1.327 (0.887-1.985) | 0.168 | ||
| Menthol aminotransferase (≥45 U/l/<45 U/l) | 0.876 (0.579-1.327) | 0.533 | ||
| Alpha-fetoprotein (≥400 mg/l/<400 mg/l) | 0.739 (0.494-1.100) | 0.133 | ||
| Glutaminase (≥40 U/l/<40 U/l) | 1.344 (0.903-1.999) | 0.145 | ||
Table 4: Unifactorial and multifactorial analysis of PFS
PHC ranks 4th among malignant tumors in China and is the second leading cause of tumor-related deaths, posing a major threat to public health. HCC accounts for 75 % to 85 % of all PHCs and is the most common type of primary liver malignancy. Its major risk factors include viral hepatitis, alcoholism, obesity and diabetes. Unfortunately, most patients are diagnosed when the disease has already progressed to intermediate to advanced stages, and the 5 y survival rate is extremely low, ranging from 10 % to 18 %[13,14]. Sorafenib has long been the only firstline drug option in the strategy for the treatment of moderately advanced HCC, and although it provides some survival benefit, the overall tumor response rate is low. Recently, significant progress has been made in the field of pharmacologic therapy for patients with intermediate and advanced-stage HCC as new drugs continue to be developed. In particular, the combined use of molecularly targeted therapies and immunotherapy has generated widespread interest, and this combination regimen is expected to significantly improve efficacy compared to monotherapy strategies[15,16]. However, despite the fact that molecularly targeted agents have demonstrated greater potential in clinical treatment, their ultimate clinical benefit remains limited due to the high degree of tumor heterogeneity and the potential for drug resistance to develop over time[17]. This point highlights the urgent need to adopt combination therapeutic strategies to enhance efficacy, through which multiple pathways of tumor growth and survival can be attacked more comprehensively with a view to achieving better therapeutic outcomes.
Anti-angiogenic therapy targeting Vascular Endothelial Growth Factor/ Vascular Endothelial Growth Factor Receptor (VEGF/VEGFR) can effectively inhibit tumor growth and metastasis[18]. In addition, drugs that inhibit angiogenesis also possess immunomodulatory effects, which can enhance the activity of T cells and promote their infiltration in tumors[19]. By promoting vascular normalization, such drugs are also able to reduce hypoxia in the tumor region, improve drug delivery efficiency, and further promote the penetration of immune cells, thereby transforming the otherwise immunosuppressive tumor microenvironment into an immune-activated state and enhancing the anti-tumor immune response[20]. The results of the studies of IMbrave 150[21,22] and ORIENT-32[23] recommended that atelizumab in combination with bevacizumab (AteBev regimen) and sindilizumab in combination with bevacizumab biosimilar (IBI305) as first-line therapeutic regimens for unrespectable HCC, and these combination regimens significantly improved patient’s OS and PFS, stimulating interest in further research on other potential combination therapies. In particular, the karelizumab in combination with apatinib regimen demonstrated significant efficacy in a phase II clinical trial (RESCUE) conducted in China, with a median PFS of 5.7 mo, an ORR of 34.3 %, and a 12 mo survival rate of up to 74.7 % in the first-line treatment cohort. The second-line cohort had a median PFS of 5.5 mo, ORR of 22.5 %, and a 12 mo survival rate of 68.2 %. The RESCUE study provided a new perspective on the systemic treatment of advanced HCC, with the combination of karelizumab and apatinib, also known as the “Double AI” program, demonstrating significant efficacy (also known as the “Double AI” regimen) has demonstrated remarkable efficacy. This regimen not only increased the response rate to treatment, but also improved patient survival while maintaining a manageable safety profile. Thus, this therapeutic strategy opens up a broader range of drug treatment options for patients with advanced HCC and provides strong clinical evidence of treatment, especially in cases where existing treatment options have limited or no effect, offering new hope to patients.
The global multicenter, randomized controlled phase III study (SHR-1210-III-310) led by Prof. Qin [24] further confirmed that this combination regimen has significant clinical benefit and acceptable safety profile in the first-line treatment of advanced HCC, with a median OS of 22.1 mo and a median PFS of 5.6 mo, which significantly reduced the risk of disease progression or death. Based on these findings, the 2022 edition of the Chinese Society of Clinical Oncology (CSCO) guidelines for the diagnosis and treatment of PHC has included the karelizumab in combination with apatinib regimen as a first-line treatment option for HCC[25]. The preliminary results of this study showed that there was no statistically significant difference in the objective remission rate and DCR between the two groups at 1 mo after treatment, and the DCR between the two groups at 3 mo after treatment. This may be due to the fact that karelizumab requires a certain time and dose to activate the body’s immune system. However, the difference was statistically significant when comparing the objective remission rate at 3 mo after treatment, objective remission rate at 6 mo after treatment, and disease remission rate in the combination group. Consistent with the results of the above study, it confirms the effectiveness of the karelizumab combined with apatinib regimen in the treatment of intermediate and advanced HCC.
This study showed that no treatment-related deaths occurred in either group during the treatment received. Treatment-induced adverse reactions were mainly concentrated in grade 1 and 2, and these symptoms were effectively relieved after receiving the appropriate symptomatic treatment, without causing harm to the patients. The difference in the incidence of adverse reactions between the two groups of patients was not statistically significant (p>0.05).
Among them, the incidence of cutaneous capillary hyperplasia in group A was lower than that in group B, similar to the results of Xu et al.[26]. The appearance of Reactive Cutaneous Capillary Endothelial Proliferation (RCCEP) may be due to the fact that karelizumab, through over-activation of the human immune function, disrupts the balance between vascular growth-promoting factors and vascular growth-inhibiting factors in the skin tissues, which promotes the proliferation of capillary endothelial cells. Comparatively, apatinib, as an anti-angiogenic drug, can reduce the incidence of skin capillary hyperplasia to a certain extent. In addition, the reason for the occurrence of grade III hypertension may be related to the underlying diseases such as hypertension that existed in some patients themselves and after being affected by the drug, their blood pressure values increased significantly compared with those before the drug was administered. For patients who developed grade III hypertension, blood pressure could be effectively controlled to below 140/90 mmHg by combining two antihypertensive drugs and appropriately adjusting the drug dosage, and this therapeutic measure did not affect the normal use of anticancer drugs. This study showed that by the end of follow-up, 4 patients in group A did not reach OS, and 8 patients in group B did not reach OS, with a median OS of 15.00 (95 % CI: 11.66- 18.34) in group A, and a median OS of 10.00 (95 % CI: 8.02-11.98) in group B (p=0.000). One patient in each of the two groups did not reach PFS at the end of follow-up. The median time to PFS was 8.0 (95 % CI: 6.69~9.31) in group A, and the median time to PFS was 5.0 (95 % CI: 2.70~7.298) mo in group B, (p=0.000). It indicated that karelizumab in combination with abatinib could alleviate the disease progression of intermediate- and advancedstage HCC and prolong the patient’s survival time. The results of Cox multifactorial analysis showed the treatment modality, Child grading was an independent prognostic factor associated with PFS in patients with intermediate and advanced HCC (p<0.05). It suggests that karelizumab combined with apatinib treatment and Child-Pugh classification A are protective factors for PFS, which can lead to a better survival advantage.
This study, as a retrospective observational study, is not a Randomized Controlled Trial (RCT), and its results may be affected by confounding bias. This means that although the study provides important insights and trends, its conclusions may be less reliable than those of a RCT. In addition, the study was based on a smaller sample size of patient population in a single center, which may limit the generalizability and replication of the results. The short duration of follow-up is also a limitation of the study, which may affect the assessment of the long-term effects and safety of the treatment. To overcome these limitations, future studies need to be conducted by means of multicenter, randomized controlled clinical trials, which will help provide a higher level of evidence-based medicine and ensure the reliability of conclusions. Conducting such studies will not only validate the validity and feasibility of current findings, but also suggest more comprehensive and innovative treatment options for patients with intermediate to advanced HCC. Through this approach, the impact of different treatments on patient survival, quality of life, and treatment-related adverse effects can be better understood, with the ultimate goal of providing more effective and safer treatment options for patients with mid- to advancedstage HCC.
In conclusion, karelizumab combined with apatinib for the treatment of intermediate and advanced HCC has a high objective remission rate and a good safety profile, which lays the foundation for the development of subsequent clinical trials.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhang R, Gao X, Zuo J, Hu B, Yang J, Zhao J, et al. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci 2020;111(2):406-17.
[Crossref] [Google Scholar] [PubMed]
- Wu M, Miao H, Fu R, Zhang J, Zheng W. Hepatic stellate cell: A potential target for hepatocellular carcinoma. Curr Mol Pharmacol 2020;13(4):261-72.
[Crossref] [Google Scholar] [PubMed]
- Moriya T, Kawakami N, Wakai Y, Saito H, Saito K. Pulmonary lipiodol embolism following transcatheter arterial chemoembolization. Respirol Case Rep 2020;8(9).
[Crossref] [Google Scholar] [PubMed]
- Terasawa M, Allard MA, Golse N, Cunha AS, Cherqui D, Adam R, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization vs. portal vein embolization alone before major hepatectomy for patients with large hepatocellular carcinoma: An intent-to-treat analysis. Surgery 2020;167(2):425-31.
[Crossref] [Google Scholar] [PubMed]
- Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: Current progress and future directions. Hepatology 2014;60(5):1776-82.
[Crossref] [Google Scholar] [PubMed]
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19(7):940-52.
[Crossref] [Google Scholar] [PubMed]
- Kudo M. Limited impact of anti-PD-1/PD-L1 monotherapy for hepatocellular carcinoma. Liver Cancer 2020;9(6):629-39.
[Crossref] [Google Scholar] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492-502.
[Crossref] [Google Scholar] [PubMed]
- Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancer 2020;12(5):1089.
[Crossref] [Google Scholar] [PubMed]
- Expert Committee for the Preparation of the Diagnostic and Treatment Guidelines for Primary Liver Cancer (Annual Edition). Diagnostic and therapeutic code for primary liver cancer (2019 edition). Chin Clin Med 2020;27(1):140-56.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52-60.
[Crossref] [Google Scholar] [PubMed]
- Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE-version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr 2021;112(1):90-2.
[Crossref] [Google Scholar] [PubMed]
- De Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 2015;62(4):1190-200.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA Cancer J Clin 2018;68(1):7-30.
[Crossref] [Google Scholar] [PubMed]
- Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6(5):555-67.
[Crossref] [Google Scholar] [PubMed]
- Hilmi M, Neuzillet C, Calderaro J, Lafdil F, Pawlotsky JM, Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: Current knowledge and future research directions. J Immunother Cancer 2019;7:1-3.
[Crossref] [Google Scholar] [PubMed]
- El Dika I, Khalil DN, Abou-Alfa GK. Immune checkpoint inhibitors for hepatocellular carcinoma. Cancer 2019;125(19):3312-9.
- Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett 201928;460:1-9.
[Crossref] [Google Scholar] [PubMed]
- Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol 2019;12:1-1.
[Crossref] [Google Scholar] [PubMed]
- Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol 2018;9:350582.
[Crossref] [Google Scholar] [PubMed]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382(20):1894-905.
[Crossref] [Google Scholar] [PubMed]
- Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) vs. sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22(7):977-90.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res 2021;27(4):1003-11.
[Crossref] [Google Scholar] [PubMed]
- Qin S, Chan LS, Gu S. Camrelizumab plus rivoceranib vs. sorafenib as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol 2022;33(7): 1401-2.
- Chinese Society of Clinical Oncology (CSCO). Guidelines for the diagnosis and treatment of primary liver cancer. Beijing: People's Publishing House; 2022.
- Xu B, Sun HC. Camrelizumab: An investigational agent for hepatocellular carcinoma. Expert Opin Investig Drugs 2022;31(4):337-46.
[Crossref] [Google Scholar] [PubMed]
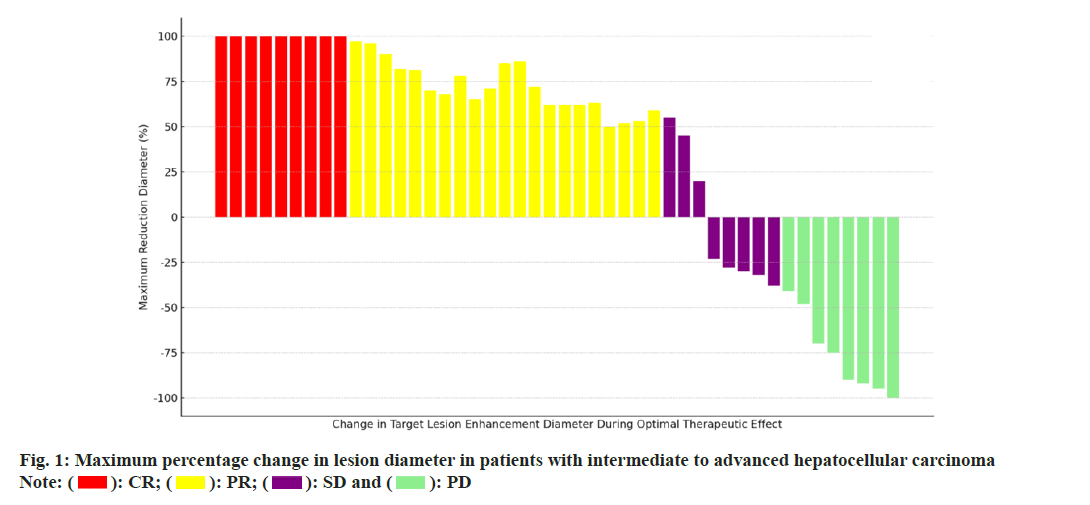
 ): CR; (
): CR; ( ): PR; (
): PR; ( ): SD and (
): SD and ( ): PD
): PD