- *Corresponding Author:
- Cheng Liang
Department of Neurology, Lanzhou University Second Hospital, Lanzhou, Gansu 730000, China
E-mail: sfq729152728@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “29-35” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acute cerebrovascular diseases are a group of common clinical diseases characterized by abnormal cerebrovascular circulation. Among many common and frequently occurring diseases admitted to emergency and critical care departments, the mortality rate is high and the disability rate of surviving patients is also high. In addition to central injury in acute cerebrovascular diseases, which can lead to poor prognosis in patients, fluctuations in the properties and functions of local and circulating immune mediators can also trigger the body to enter an immune imbalance state, leading to poor prognosis in some patients after receiving systematic symptomatic support treatment. Betahistine hydrochloride is a histamine H1 receptor agonist that can resist platelet aggregation. The drug has a half-life of 5 h through liver metabolism and will not remain in the body, resulting in high safety. In addition, betahistine hydrochloride also has a diuretic effect, which can promote blood circulation, increase organ blood perfusion, reduce myocardial oxygen consumption, improve patient blood pressure levels and ultimately improve symptoms of cardiovascular and cerebrovascular ischemia and hypoxia. Therefore, this article mainly reviews the treatment of acute cerebrovascular disease with betahistine hydrochloride combined with atorvastatin, providing scientific reference for clinical treatment of acute cerebrovascular disease.
Keywords
Betahistine hydrochloride, atorvastatin, acute cerebrovascular disease, procalcitonin, C-reactive protein
Cerebrovascular diseases have become the second leading cause of death in the world. Cerebrovascular disease is characterized by high incidence rate, high recurrence rate, high disability rate and high mortality[1]. With the aging population and significant improvement of living standards in China, the incidence of cerebrovascular diseases has increased at an average annual rate of 8.3 %, ranking first among the causes of death among Chinese residents and being the main cause of illness and death in patients with the nervous system[2]. The incidence of acute severe cerebrovascular disease is also increasing year by year with the increasing incidence of cerebrovascular diseases, which is the focus of attention in the medical community. Middle aged and elderly people are the people with high incidence of acute cerebrovascular diseases. Recent surveys show that their incidence rate is increasing year by year and patients are getting younger and younger, which has become one of the main causes of human death[3,4]. Cerebral infarction is an ischemic stroke, accounting for 70 % of all acute cerebrovascular diseases in incidence rate[5,6]. Clinical treatment of cerebrovascular diseases usually involves controlling the condition through thrombolysis, anticoagulation and repair of damaged nerves, but there is still a lack of specific drugs and related treatment plans. Betahistine hydrochloride is a Histamine (H1) receptor agonist that can resist platelet aggregation. The drug has a half-life of 5 h through liver metabolism and will not remain in the body, resulting in high safety. In addition, betahistine hydrochloride also has a diuretic effect, which can promote blood circulation, increase organ blood perfusion, reduce myocardial oxygen consumption, improve patient blood pressure levels and ultimately improve symptoms of cardiovascular and cerebrovascular ischemia and hypoxia. In this article, we review the progress in the treatment of acute cerebrovascular disease patients using betahistine hydrochloride combined with atorvastatin.
Current Research Status of Acute Cerebrovascular Disease
With the reform and opening up and the arrival of the era of “Internet plus”, China’s various sectors have witnessed unprecedented rapid development. The traditional sales concept and fixed job model have been broken. The era of one person holding multiple jobs and cross bank robbery has become a trend of social development, which has led to a fast pace of life, increased pressure, a general increase in the incidence of various diseases, and the problem of food and clothing for people has been solved. The variety of diet is becoming more and more abundant, the diet structure of life has changed dramatically, the incidence rate of cerebrovascular disease has increased year by year, its disease progress is fast and the disability rate is high, so it has become the "first key" to threaten people’s health[2]. In the latest national survey on the causes of death of residents, the health department found that the total death toll of acute cerebrovascular disease was about (136/100 000), which has far exceeded lung cancer, esophageal cancer, breast cancer and other malignant tumor diseases and ranked first in the overall cause of death in China. As it is well known, according to the anatomical characteristics, pathological properties and pathological and physiological evolution of cerebral blood vessels, acute cerebrovascular disease often leads to physical disability. The increasing variety of diets, excessive intake of nutrients and the arrival of the era of using cars as a means of transportation have led to an increasing incidence of acute cerebrovascular disease year by year, causing medical units of all sizes in China to continuously expand their beds to meet the basic medical needs of the people. As a result, it has greatly caused the loss of national resources and the heavy medical burden and suffering of their own families. According to statistics, the losses caused to the national economy can reach as high as 27 to 200 billion Yuan per year[7,8]. As the saying goes, “time is the brain”. If patients with acute cerebrovascular disease can enter the “stroke unit” for standardized diagnosis and treatment in the shortest possible time, it will greatly reduce the disability rate. The most important therapeutic target of acute cerebrovascular disease is to save the brain tissue that is on the verge of ischemic necrosis, that is, the "ischemic penumbra". In recent years, the prevailing intravascular interventional therapy and the "alteplase" thrombolytic therapy in the hyperacute phase have become the most effective treatment means[9]. It is greatly beneficial for improving disability, healing, reducing post ischemic reperfusion injury, secondary bleeding and brain tissue edema.
Current Clinical Treatment Status of Acute Cerebrovascular Disease
Antiplatelet therapy:
Antiplatelet therapy has important value in the treatment of acute cerebrovascular disease. It has significant effect in inhibiting platelet aggregation and through this mechanism, it can effectively prevent thrombosis and effectively reduce the risk of cerebrovascular disease. Related drugs mainly include glycoprotein Ⅱ b/Ⅲ a receptor antagonists (including abciximab, tirofiban, etc.), dipyridamole, clopidogrel, apixaban, aspirin, ticlopidine, etc. Among them, aspirin is a commonly used and proven as effective drug in clinical practice. For patients with acute ischemic cerebrovascular disease (within 48 h of onset), aspirin has been proven to reduce secondary recurrence, effectively reduce disability and mortality rates. However, it should be noted that thrombolysis and anticoagulation therapy cannot be used simultaneously, otherwise it will increase the risk of intracranial hemorrhage. Clopidogrel has a more prominent antiplatelet effect compared to aspirin and has important value in preventing and controlling ischemic stroke. The existing research also confirmed that[10], the use of ticlopidine is better than aspirin in reducing the mortality and recurrence rate, and has important application value in the prevention and treatment of stroke.
Thrombolytic therapy:
Thrombolytic therapy refers to the treatment of cerebrovascular diseases with thrombolytic agents placed within the effective time window (usually considered within 3-6 h), which has been proven to be a specific drug therapy for such diseases[11]. Thrombolytic therapy can be divided into two pathways based on the actual placement position-Arterial and venous thrombolysis, and the combination of arterial and venous thrombolysis can also achieve ideal therapeutic effects. Intravenous thrombolytic therapy is mostly used for remote vascular recanalization. Thrombolytic agents mainly include Streptokinase (i.e. SK), Urokinase (i.e. UK) and recombinant tissue-Plasmin Activator (rt- PA). Among them, rt-PA has been approved by the United States (US) Food and Drug Administration, playing an important role in the treatment of acute cerebrovascular disease and has become the main drug for clinical treatment of this type of disease[12]. Compared with other thrombolytic agents (such as SK and UK), rt-PA has significant advantages, especially in improving recanalization rate and reducing the risk of intracranial hemorrhage, and can effectively prevent the occurrence of intracranial hemorrhage. A study has found that intravenous thrombolysis with UK is effective and safe for patients with acute cerebrovascular disease within 6 h of onset. However, caution should be exercised in drug dosage (especially when used at high doses), as there may be a risk of disability or even death. Especially when high-dose use of urease increases, the risk of intracranial hemorrhage increases and even leads to patient death and they are limitations to clinical application. At the same time, attention should be paid to the important factor of treatment time window, which is also one of the key factors affecting the efficacy of thrombolysis in patients with cerebrovascular diseases. It is generally believed that the optimal treatment time window is to carry out thrombolysis treatment within 3 h and to carry out thrombolysis treatment within 6 h which is an effective treatment time window. Research has confirmed that rt-PA thrombolysis therapy has the best effect when the time window is 4.5 h [13].
The Relationship Between Procalcitonin (PCT) and Acute Cerebrovascular Disease
PCT is a physiological glycoprotein that is mainly produced by thyroid C cells and has no hormonal activity. After bacterial infection, macrophages (originating from the liver), monocytes (originating from the liver), lymphocytes (originating from lung and intestinal tissues), etc. will synthesize and secrete PCT. Endotoxins have been proven to trigger inflammatory response syndrome (with a higher incidence of acute cerebral infarction and poor prognosis) and multiple organ dysfunction syndrome (with a risk of acute cerebrovascular disease leading to poor prognosis). In a systemic inflammatory response state, the nervous system is activated to release various cytokines and neurohormones, which may lead to a cascade reaction that seriously damages the body’s health[14]. Under normal circumstances, the concentration of PCT in human peripheral blood is extremely low, even undetectable and significantly increases during infection. PCT concentration is closely related to the scope and degree of infection.
The Relationship Between C-Reactive Protein (CRP) and Acute Cerebrovascular Disease
CRP is a non-specific and important sensitive inflammatory marker, and its level rapidly increases when detected after the body experiences inflammatory reactions or tissue damage. It has been confirmed that its content is very low in healthy individuals, but CRP can increase several times or even up to 100 times in a short period of time after tissue damage. Therefore, CRP is a very common and important inflammatory marker in clinical practice[15]. Multiple studies have confirmed that CRP is involved in the pathogenesis of coronary heart disease, acute cerebral infarction, cerebral hemorrhage and other diseases. The mechanism of its involvement in the occurrence and development of these diseases may be that after tissue damage, CRP can bind to endothelial cells in the damaged area, regulate phagocytosis by clearing foreign pathogenic factors or activating complement and promote the release of various cytokines by immune cells in the body alone or in collaboration with other substances. To a large extent, it exacerbates the damage to vascular endothelial cells, thereby promoting the occurrence and development of related diseases[16]. Other studies have found that serum CRP levels are important indicators for evaluating and predicting the severity and prognosis of acute cerebrovascular disease patients, and their levels are positively correlated with the severity of the above patient’s conditions[17,18]. At present, it is widely believed that although CRP levels increase after detection without specificity, they have important value in evaluating the efficacy of various inflammatory reactions, bacterial infections and cerebrovascular disease patients.
Pharmacological Effects of Betahistine Hydrochloride
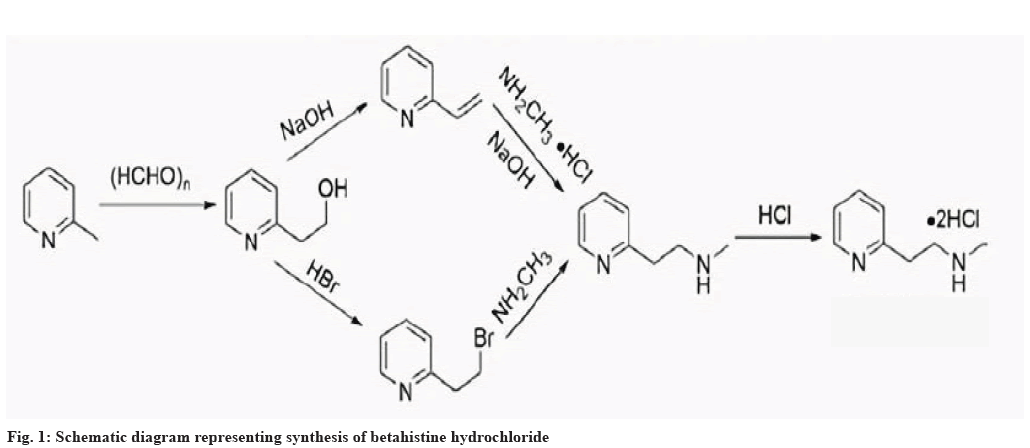
Betahistine hydrochloride is a histamine H1 receptor agonist (belonging to the category of histamine like drugs), used clinically as a vasodilator to treat various dizziness syndromes, chronic ischemic cerebrovascular diseases, Meniere’s syndrome and related dizziness symptoms. Especially in the treatment of Meniere’s syndrome, betahistine hydrochloride is an important and commonly used drug, with definite efficacy and high safety[19]. Previous studies have shown that betahistine hydrochloride can exert therapeutic effects by binding to vascular smooth muscle H1 receptors, effectively increasing blood flow to cardiovascular and cerebrovascular tissues, as well as adjacent vascular tissues. It can also improve microcirculation to a certain extent, effectively reducing platelet adhesion rate and red blood cell aggregation. Therefore, it can effectively improve blood viscosity, alleviate blood hypercoagulability in patients with cerebrovascular diseases and be absorbed quickly after oral administration. The overall efficacy is accurate and safe. After entering the body, the metabolites of betastatin are 2-pyridylacetic acid, 2-pyridylethylamine, etc. The former (i.e. 2-pyridylacetic acid) is mostly excreted through urine and research has found that its metabolites can be completely excreted within 24 h[20]. Existing evidence suggests that 2-pyridylethylamine, as a metabolite of betahistine, may also have activity and play a similar role on ampullary receptors as betahistine hydrochloride. Schematic diagram of synthesis route of betahistine hydrochloride is shown in fig. 1.
Clinical Application Status of Betahistine Hydrochloride in Acute Cerebrovascular Disease
Liu et al.[21] selected 80 hypertensive cerebral vasospasm patients and randomly divided them into two groups. Stop the use of all antihypertensive drugs 1 w before enrollment. The control group received nimodipine tablets, while the observation group received betahistine on this basis. The results showed that the index of cerebral vasospasm in the observation group was significantly lower than that in the control group 3 d, 1 w and 1 mo after treatment (p<0.05), compared with the control group, which was significantly reduced (p<0.05). After treatment, the level of Endothelin 1 (ET-1) in the observation group was significantly lower than that in the control group, suggesting that the above scheme is helpful to improve the vascular endothelial function in patients with hypertensive cerebral vasospasm. Further study found that cerebral vasospasm index and high sensitivity C-Reactive Protein (hsCRP), Interleukin-6 (IL-6), Tumor Necrosis Factor alpha (TNF-α) was also lowered. There was a positive correlation between ET-1 and ET-1 (p<0.05), but a negative correlation between ET-1 and NO (p<0.05). The effective rate of blood pressure regulation and treatment of cerebral vasospasm in the observation group was significantly higher than that in the control group (p<0.05) and the group did not increase adverse reactions. It can be seen that the combination of betahistine and nimodipine in the treatment of hypertensive cerebral vasospasm can effectively reduce the aseptic inflammatory reaction of the body, improve vascular endothelial function, increase cerebral circulation blood flow and provide a favorable strategy for clinical treatment. Meng et al.[22] said that betahistine hydrochloride can effectively improve arterial hemodynamics and cerebral blood flow perfusion in patients with vertebrobasilar artery insufficiency, improve clinical efficacy and has high safety, which is worth extensive application.
Pharmacological Effects of Atorvastatin
Atorvastatin is an important and widely used lipid-lowering drug, with a structure similar to 3-Hydroxymethyl-3-Methylglutaryl-Coenzyme A (HMG-CoA), but much higher affinity than the latter. Atorvastatin can competitively inhibit HMGCoA reductase to a certain extent and affect the synthesis of cholesterol in the liver. This mechanism of action downregulates plasma cholesterol levels, thereby exerting its lipid-lowering effect[23,24]. Once administered, atorvastatin can affect the surface receptors of liver cells, promote their uptake of low-density lipoprotein and thus play a role in accelerating catabolism and lowering blood lipids. Atorvastatin is also suitable for patients with various types of hypercholesterolemia (familial, non-familial or mixed type) and lipid metabolism disorders, effectively reducing plasma levels of total cholesterol, triglycerides and low-density lipoprotein, and exerting a lipid-lowering effect.
Current Status of Clinical Application of Atorvastatin
Anti-atherosclerosis effect:
Atorvastatin has been confirmed by multiple studies to have significant antioxidant effects, helping to stimulate Nitric Oxide Synthase (NOS) activity, block Renin-Angiotensin-Aldosterone System (RAAS) and thus affect endothelial function. Other studies have confirmed that atorvastatin also plays an important role in improving hypertension caused by Angiotensin II receptor Type 2 (AT2) injection and helps to reduce AT1 receptor density. In addition, atorvastatin can also inhibit various effects such as nitrogen oxides and Rac isopentenylation, thus inhibiting oxidative stress reactions. Atorvastatin can significantly enhance its antioxidant capacity by inhibiting Angiotensin II receptor Type 1 (AT1), increasing the uptake of low-density lipoprotein receptors and promoting the production of nitric oxide, thereby controlling the formation of oxygen free radicals[25].
Lowering blood pressure:
Atorvastatin also has important application value in lowering blood pressure. Previous studies have confirmed that it can reduce multiple indicators such as serum total cholesterol, low-density lipoprotein cholesterol and hypersensitive CRP levels, and can increase adiponectin levels, but it does not affect soluble Cluster of Differentiation 40 (CD40) ligands[26,27]. In clinical practice, patients with hyperlipidemia combined with hypertension were treated with a combined treatment scheme (namely, atorvastatin, amlodipine, rosiglitazone and Tianma- Zexie decoction, a traditional Chinese medicine prescription), which can achieve significant results. Some domestic scholars have observed the effect of amlodipine combined with multiple drugs in the treatment of primary hyperlipidemia with hypertension. The results show that the effect of the combined drug group is significantly better than that of the control group given amlodipine conventional treatment and it is safe and reliable.
Treatment of coronary heart disease and angina pectoris:
Many existing studies have confirmed that atorvastatin can effectively control and reduce the level of low-density lipoprotein cholesterol[28,29], which has important value in systolic heart failure, atherosclerosis and controlling the progression of sclerotic plaque lesions. At present, the academic community believes that atorvastatin has a significant effect on regulating high and low density lipoprotein cholesterol levels in patients with coronary heart disease and angina pectoris, and can affect important indicators such as lactate dehydrogenase, creatinase isoenzyme and hydroxybutyrate dehydrogenase phosphate levels. However, its effectiveness has been confirmed by previous relevant studies to be inferior to the combination of other drugs (such as tanshinone IIA sulfonate sodium), indicating that the combination of drugs for coronary heart disease treatment and Angina pectoris can achieve more ideal results.
Conclusion
As it is well known, acute cerebrovascular disease is a sudden onset that leads to functional deficits in the corresponding regions of the brain. Due to its high number of patients, deaths, relapses and disabilities, it has become one of the important factors seriously endangering people’s physical health and quality of life. In recent years, the incidence rate of acute cerebrovascular disease has increased significantly in China and even around the world due to the aging population, increasing life pressure, lifestyle changes, diet adjustment and other complex factors. How to effectively prevent and treat acute cerebrovascular disease has become an important issue of concern to relevant researchers and further in-depth research should be carried out in the future to explore more ideal, safe and suitable drug treatment plan for acute cerebrovascular disease, exploring the feasibility and safety of combined medication treatment, in order to improve the treatment effect and prognosis of patients with acute cerebrovascular disease.
Funding:
This study was supported by Gansu Provincial Natural Science Foundation (No.: 22JR5RA1008).
Conflict of interests:
The authors declared no conflict of interest.
References
- Goldstein LB. Introduction for focused updates in cerebrovascular disease. Stroke 2020;51(3):708-10.
[Crossref] [Google scholar] [PubMed]
- Santos M, de Sousa DA. Cerebrovascular disease in pregnancy and postpartum. Curr Opin Neurol 2022;35(1):31-8.
[Crossref] [Google scholar] [PubMed]
- Kelly DM, Ademi Z, Doehner W, Lip GY, Mark P, Toyoda K, et al. Chronic kidney disease and cerebrovascular disease: Consensus and guidance from a KDIGO controversies conference. Stroke 2021;52(7):e328-46.
[Crossref] [Google scholar] [PubMed]
- Marini S, Merino J, Montgomery BE, Malik R, Sudlow CL, Dichgans M, et al. Mendelian randomization study of obesity and cerebrovascular disease. Ann Neurol 2020;87(4):516-24.
[Crossref] [Google scholar] [PubMed]
- Narasimhan M, Schwartz R, Halliday G. Parkinsonism and cerebrovascular disease. J Neurol Sci 2022;433:120011.
[Crossref] [Google scholar] [PubMed]
- Fernández-Eulate G, Arocena P, Muñoz-Lopetegi A, Rodriguez-Antigüedad J, Campo-Caballero D, Equiza J, et al. Attention to acute cerebrovascular disease in Guipúzcoa: Description of the results of a reference hospital in a centralized care model. Neurología 2022;37(5):355-61.
[Crossref] [Google scholar] [PubMed]
- Patrick L. Headache and acute cerebrovascular disease: How do we differentiate primary and secondary headache disorders in the emergency setting? Headache 2022;62(9):1073-4.
[Crossref] [Google scholar] [PubMed]
- Al-Kawaz M, Cho SM, Gottesman RF, Suarez JI, Rivera-Lara L. Impact of cerebral autoregulation monitoring in cerebrovascular disease: A systematic review. Neurocrit Care 2022;36(3):1053-70.
[Crossref] [Google scholar] [PubMed]
- Shaban S, Huasen B, Haridas A, Killingsworth M, Worthington J, Jabbour P, et al. Digital subtraction angiography in cerebrovascular disease: Current practice and perspectives on diagnosis, acute treatment and prognosis. Acta Neurol Belg 2022;122(3):763-80.
[Crossref] [Google scholar] [PubMed]
- Sharma R, Kumar P, Prashanth SP, Belagali Y. Dual antiplatelet therapy in coronary artery disease. Cardiol Ther 2020;9:349-61.
[Crossref] [Google scholar] [PubMed]
- Grotta JC. Intravenous thrombolysis for acute ischemic stroke. Continuum 2023;29(2):425-42.
[Crossref] [Google scholar] [PubMed]
- Chun TT, O’Connell JB, Rigberg DA, deRubertis BG, Jimenez JC, Farley SM, et al. Preoperative thrombolysis is associated with improved vein patency and functional outcomes after first rib resection in acute Paget-Schroetter syndrome. J Vasc Surg 2022;76(3):806-13.
[Crossref] [Google scholar] [PubMed]
- Tian D, Zhang S, He X, Liu H. Serum procalcitonin as a diagnostic marker in acute ischemic stroke. Neuroreport 2015;26(1):33-7.
[Crossref] [Google scholar] [PubMed]
- Deng WJ, Shen RL, Li M, Teng JF. Relationship between procalcitonin serum levels and functional outcome in stroke patients. Cell Mol Neurobiol 2015;35:355-61.
[Crossref] [Google scholar] [PubMed]
- Nurmohamed NS, Belo Pereira JP, Hoogeveen RM, Kroon J, Kraaijenhof JM, Waissi F, et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. Eur Heart J 2022;43(16):1569-77.
[Crossref] [Google scholar] [PubMed]
- Sheriff A, Kayser S, Brunner P, Vogt B. C-reactive protein triggers cell death in ischemic cells. Front Immunol 2021;12:630430.
[Crossref] [Google scholar] [PubMed]
- Li S, Jing J, Li J, Wang A, Meng X, Wang Y. Elevated hs-CRP and symptomatic intracranial/extracranial artery stenosis predict stroke recurrence after acute ischemic stroke or TIA. J Atheroscler Thromb 2023;30(6):601-10.
[Crossref] [Google scholar] [PubMed]
- Li K, Zhang Q, Lu X, Yao S. Effects of butylphthalide sodium chloride injection combined with edaravone dexborneol on neurological function and serum inflammatory factor levels in sufferers having acute ischemic stroke. J Healthc Eng 2022;2022:1-6.
[Crossref] [Google scholar] [PubMed]
- van Esch B, van der Zaag-Loonen H, Bruintjes T, van Benthem PP. Betahistine in Menière’s disease or syndrome: A systematic review. Audiol Neurootol 2022;27(1):1-33.
[Crossref] [Google scholar] [PubMed]
- Wu P, Cao W, Hu Y, Li H. Effects of vestibular rehabilitation, with or without betahistine, on managing residual dizziness after successful repositioning manoeuvres in patients with benign paroxysmal positional vertigo: A protocol for a randomised controlled trial. BMJ Open 2019;9(6):e026711.
[Crossref] [Google scholar] [PubMed]
- Liu X, Zhao NN, Zeng K, Xiao P, Sheng P, Luo X, et al. Effects of nimodipine combined with betahistine on CRP and other inflammatory cytokines and vascular endothelial function in patients with hypertensive cerebral vasospasm. Clin Hemorheol Microcirc 2020;75(3):279-89.
[Crossref] [Google scholar] [PubMed]
- Meng S, Liu Q, Zhang L. Clinical efficacy and safety of flunarizine tablets combined with betahistine hydrochloride tablets in patients with vertebrobasilar insufficiency vertigo. Am J Transl Res 2022;14(11):8183-90.
[Google scholar] [PubMed]
- Taniguti EH, Ferreira YS, Stupp IJ, Fraga-Junior EB, Doneda DL, Lopes L, et al. Atorvastatin prevents lipopolysaccharide-induced depressive-like behaviour in mice. Brain Res Bull 2019;146:279-86.
[Crossref] [Google scholar] [PubMed]
- Jiang R, Zhao S, Wang R, Feng H, Zhang J, Li X, et al. Safety and efficacy of atorvastatin for chronic subdural hematoma in Chinese patients: A randomized clinical trial. JAMA Neurol 2018;75(11):1338-46.
[Crossref] [Google scholar] [PubMed]
- Zhang L, Zhang S, Yu Y. Efficacy and safety of rosuvastatin vs. atorvastatin in lowering LDL cholesterol: A meta-analysis of trials with East Asian populations. Herz 2020;45(6):594-602.
[Crossref] [Google scholar] [PubMed]
- Perez-Calahorra S, Laclaustra M, Marco-Benedi V, Pinto X, Sanchez-Hernandez RM, Plana N, et al. Comparative efficacy between atorvastatin and rosuvastatin in the prevention of cardiovascular disease recurrence. Lipids Health Dis 2019;18(1):1-6.
[Crossref] [Google scholar] [PubMed]
- Liping Z, Xiufang L, Tao Y, Baomin Z, Houshuai T. Efficacy comparison of rosuvastatin and atorvastatin in the treatment of atherosclerosis and drug safety analysis. Pak J Pharm Sci 2018;31(5):2203-8.
[Google scholar] [PubMed]
- Chen J, Yan J, Li S, Zhu J, Zhou J, Li J, et al. Atorvastatin inhibited TNF-α induced matrix degradation in rat nucleus pulposus cells by suppressing NLRP3 inflammasome activity and inducing autophagy through NF-κB signaling. Cell Cycle 2021;20(20):2160-73.
[Crossref] [Google scholar] [PubMed]
- Shaghaghi Z, Alvandi M, Farzipour S, Dehbanpour MR, Nosrati S. A review of effects of atorvastatin in cancer therapy. Med Oncol 2023;40(1):1-6.
[Crossref] [Google scholar] [PubMed]