- *Corresponding Author:
- R. Kumar
School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab 144601, India
E-mail: rajesh.23035@lpu.co.in
| Date of Received | 22 December 2023 |
| Date of Revision | 26 June 2024 |
| Date of Acceptance | 01 October 2024 |
| Indian J Pharm Sci 2024;86(5):1554-1563 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bacterial infections are the most important cause of severe diseases. Antibiotics are the only major option to cure these infections. Most of the bacteria (approximately 90 %) have an extracellular polymeric substance layer forming a biofilm that allows them to resist the antimicrobial agents. It enables them to develop resistance against multiple drugs. As it is a tedious task to develop novel antimicrobial agents, there is a serious need to adopt alternative strategies. To counter the multidrug resistance in the bacteria, antimicrobial peptides have been developed and used. Antimicrobial peptides are potent antibiofilm agents possessing a promising broad spectrum of activity for the treatment of a variety of infections caused by bacteria, viruses and fungi. However, some of these peptides have a very short half-life in different species of microorganisms. Some antimicrobial peptides even produce toxic effects. Hence, there is a need to continuously develop safe and effective peptide analogues to be used as potential antibiofilm agents. The present review highlights the mechanism of the development of resistance towards antimicrobial agents and the role of antimicrobial peptides as antibiofilm agents in combating this resistance with a critical analysis of their benefits and limitations. In addition, an attempt has been made to review the latest developments on antimicrobial peptides along with their applications and patents published/granted thereon.
Keywords
Antimicrobial peptides, multidrug resistance, bacteria, biofilm, antibiofilm
Bacterial infections are the most important cause of severe diseases, whereas antibiotics are the only major option to cure these diseases. Bacteria are of 2 types’ gram positive and gram negative, both are having the ability to grow rapidly and spread and cause infection. Due to variations in growth, they are most susceptible to resisting the drug by different patterns. The SCOPE project has noticed that among all, gram-positive bacteria have caused infection for 62 % and gram-negative are responsible for 22 % of infections in which abnormal cells divide without control and can damage nearby tissues also. The well-known bacterial example of a severe disease-causing gram-positive is Staphylococcus aureus and the gram-negative is Pseudomonas aeruginosa. These are having the inherent ability to resist the drug in new ways and pass along to genetic materials that allow other bacteria to resist the drug. Pneumonia, Urinary Tract Infection (UTI), gonorrhoea, abscesses (a painful collection of pus), bloodstream infections, etc., are infections that require the inclusion of antibiotics to cure. These bacteria are imputable to matrix-enclosed layers of extracellular polymeric substances known as biofilm[1]. Biofilm formation improves the defense ability of the bacteria and improves drug tolerance which introduces a big challenge to the use of antibiotics[2]. Biofilms are responsible for biofouling, notably, it is being used to survive in harsh conditions by bacteria[3].
As antibiotics have similar spectrum activity they indicated the urgent need for novel target technologies and emerging therapeutic strategies including vaccines and target therapy for virulence factors[4]. Intriguingly Antimicrobial Peptide (AMP) caught the attention to be a potential candidate for novel drug therapy[5]. AMPs are attractive candidates to counter the effect of biofilm and can act as a potent antibiofilm agent, many of these have a broad spectrum antimicrobial activity as well as antifungal and also acts against virus[6]. AMPs also have a structural advantage as they are ubiquitous in nature. They are found in various plants, animals, viruses and bacteria as well as all mammalian species[7]. These amino acid reach molecules act as the first line of defense system against all types of pathogens separate or synergistically with the natural immune system to prevent the growth of infectious pathogens[8]. Their antimicrobial activity is better than traditional antibiotics as they can act by different mechanisms and thus can be used against microorganisms like bacteria, viruses, fungi, archaea and parasites[7].
Multidrug resistance:
Resistance has burdened the health sector as it causes the challenge to control the infection. Resistance is the ability of the microorganism which does not allow its population to react to an antimicrobial agent then it is said to be resistant[9]. Incessant and improper use of these antibiotics, and simultaneously with ineffective performance bacteria pathogens profoundly developed resistance[10]. Various mechanisms account for the emergence of resistance to the drug. Some of them are discussed below. First, they accumulate multiple genes coding for resistance entrapped in a single cell, this accumulation occurs usually on plasmids (resistance plasmids) or transposons whereas, for a specific agent, there is a separate coding. They can deplete the efficacy of many types of drugs. Second multidrug resistance occurs when genes code for drug efflux pumps are expressed more frequently, which leads to decreasing the concentration of active drugs in the body[11]. The pervasive nature of 6 bacterial species known as ESKAPE which are named Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species peculiar in multidrug resistance. And they can pass this genetic information to the next generation of microorganisms so that they are prone to develop resistance against a wider variety of drugs[12].
Biofilm:
Biofilm is a conglomerate of Extracellular Polymeric Substances (EPS) consisting of polysaccharides, proteins and other biomolecules where microbial communities are enclosed in a 3D extracellular matrix[13]. The biofilm life cycle depends upon the planktonic bacteria, which primarily attach to the surface leading to the production of EPS for the contingency of the biofilm layer and when the mature biofilm formation occurs it relocates to a new location spreading infection[14]. Biofilm disseminates antibiotic resistance in an enhanced way to make the bacterial population mutinous to host immune responses leading to continuous and tenacious spread within the host causing infections. More than 80 % of serious chronic infections are generally caused by the occurrence of biofilm on bacteria surface which results in morbidity as well as increased mortality rate also[15]. Potential characteristics of biofilms are they have novel and unique structures which are not identical and the pattern of their occurrence is not even predictable. Biofilms have emergent properties like spatial organization, host surface adhesion and cohesion and most important chemical heterogeneity leading to improved tolerance of antimicrobial agents[16]. The first appearance of biofilms was in an aquatic environment, but now they have been found in they have perceived occurring in a wide range of disease states from UTI to respiratory tract infection, etc[17]. If bacteria were able to develop a biofilm layer within a host, it will become very difficult to control the severity as bacteria will further flourish to cause a chronic infection due to biofilm[18].
AMPs:
Peptides are short protein chains of amino acid residue. They possess various properties and can be an important asset in the treatment of multidrug resistance cases. AMPs are small proteins that typically include up to 50 amino acid residues with a disulfide bridge present between them. Depending on their structure, size and orientation, they can be classified as cationic peptides and they have cationic charge ranging from +2 to +9 and have amphiphilic nature having a molecular weight of less than 10 kDa[19], which are the biggest, noncationic peptides, heterocyclic peptides, or proteins that bind oxygen. Specifically, natural peptides contain a long chain of amino acids[20]. Natural peptides show a wide spectrum of therapeutic activity acting as anti-fungal, anti-viral, anti-bacterial, etc. Arising an emergency in bacterial conditions like multi-drug resistance is very critical and life-threatening condition for the patient. Natural peptides found to be the potential to counter this and a new major challenge biofilm showing promising results than conventional antibiotics and its derivatives gave assurance to overcome multi resistant threats[20]. Sources of natural peptides are plants, animals, marine products and mammals also. The main motive for studying peptides is to treat bacterial infection and avoid challenges regarding antimicrobial resistance. The efficacy of natural AMPs is mainly based on cationic charge and amphipathic folds attached to the negatively charged membrane of microorganisms which causes disruption of cell[21].
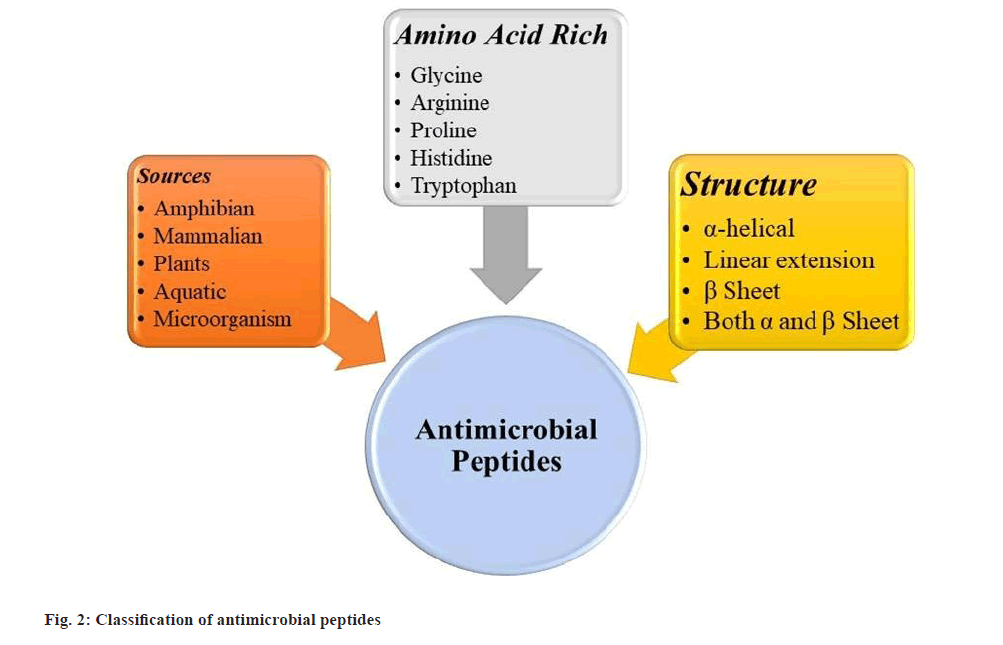
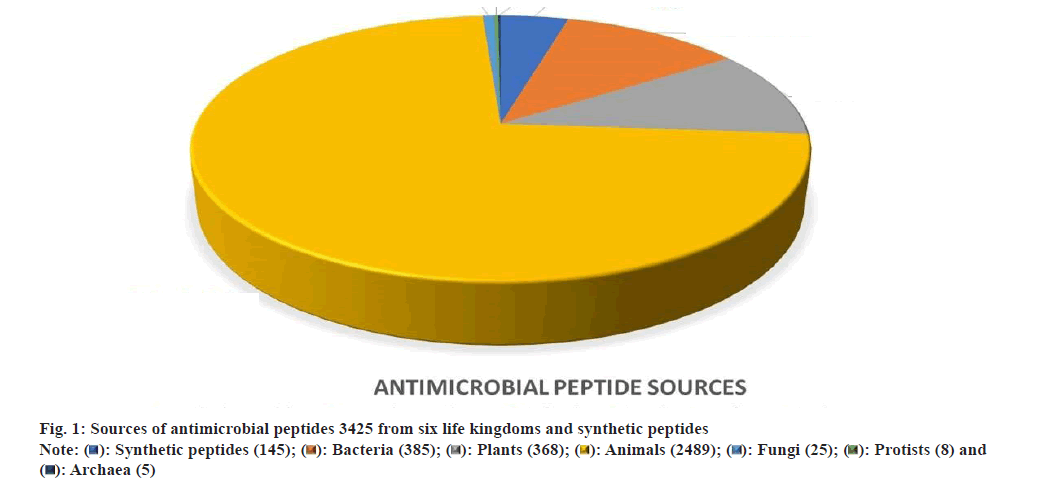
Fig. 1 has demonstrated the natural and synthetic AMPs along with their different sources. Data obtained from antimicrobial peptide database. Fig. 2 shows the classification of AMPs and their different types of structure.
Synthesis method for synthetic peptides:
Synthetic peptides and semi-synthetic peptides are of utmost interest as natural peptides have stability and purification challenges. Researchers observed that only peptides can be synthesized with a long chain of 50 amino acids. Solid-Phase Peptide Synthesis (SPPS) is a widely utilized method. For SPPS resins, linkers coupling agents and protecting groups are required. Resins impart physical and mechanical as well as chemical stability in the process. 9-fluorenylmethyloxycarbonyl is protecting group necessary to shield the functions of amino acids. A bifunctional moiety is utilized for creating a cleavable connection between peptide and resin backbone[22].
Antibiofilm peptides:
Antibiofilm peptides have an amphipathic domain. Most of the AMPs are comprised of cationic and positive net charge allowing them to act as bactericidal activity[23]. Among all α helical endowed peptides studied most by researchers. AMPs can act by both membranolytic and non-membranolytic activities including intracellular activity targeting DNA, RNA and proteins[24,25].
Table 1 show the natural AMPs available and from which sources we can obtain them along with their AMP Database ID as each peptide is of unique sequence, they are assigned with unique ID.
| Name | AMP Database ID | Source |
|---|---|---|
| Temporin B | AP00095 | European common frog, Rana temporaria |
| Indolicidin | AP00150 | Bovine neutrophils, cattle, Bos taurus[27] |
| SMAP28 | AP00155 | Sheep leukocytes, Ovis aries[41] |
| SMAP29 (Cathelicidin) | ||
| Pleurocidin | AP00166 | Skin mucous secretions, winter flounder, Pleuronectes americanus[42] |
| Protegrin 1 | AP00195 | Leukocytes; porcine neutrophil, pig, Sus scrofa[43] |
| Nisin A | AP00205 | Streptococcus lactis, reclassified as Lactococcus lactis |
| Tachyplesin III | AP00213 | Southeast Asian, Tachypleus gigas |
| Human β defensin 3 | AP00283 | Skin, tonsils, oral/saliva, colonic mucosa, Homo sapiens |
| Cathelicidn LL37 | AP00310 | Neutrophils, monocytes, mast cells, lymphocytes, mesenchymal stem cells, islets, skin, sweat, airway surface liquid, saliva, colonic mucosa, Homo sapiens, also Pan troglodytes[44] |
| Citropin 1.1 | AP00351 | Skin secretion, Australian blue mountains tree frog, Litoria citropa |
| BMAP 27 | AP00366 | Cattle, Bos taurus |
| BMAP 28 | AP00367 | Cattle, Bos taurus |
| Agelaia-MP | AP00522 | Social wasp, Agelaia pallipes |
| Human β defensin 2 | AP00524 | Skin, lung, trachea epithelia, and uterus, oral (saliva), Homo sapiens |
| Chicken cathelicidn 2 | AP00548 | chicken, Gallus gallus |
| Temporin 1CEb | AP00605 | Rana chensinensis, China, Asia |
| Temporin 10La | AP00871 | Florida bog frog, Rana okaloosae, North America |
| N atra Cathelicidn | AP00897 | Chinese cobra, Naja atra |
| Temporin PTa | AP01434 | Hylarana picturata, Asia |
| Myxinidin | AP01578 | Epidermal mucus, Myxine glutinosa L. |
| Phylloseptin 1 | AP01581 | Waxy monkey frog, Phyllomedusa sauvagei, South America |
| Polybia-MP-II | AP01640 | Venom, social wasp, Polybia paulista, Also, Pseudopolybia vespiceps testacea |
| Coprisin | AP01976 | Dung beetle, Copris tripartitus |
| Datucin | AP02065 | Datura stramonium, India, Asia |
| CCL20 | AP02075 | Skin, Homo sapiens |
| UyCT3 | AP02126 | Venom, Urodacus yaschenkoi, Australia; also Opisthacanthus cayaporum |
| Colistin A | AP02204 | Paenibacillus polymyxa var. colistinus; Also known as Bacillus polymyxa |
| TsAP-2 | AP02235 | Venom, the Brazilian yellow scorpion, Tityus serrulatus, also Turrilites costatus; Tityus obscurus, South America |
| Gramicidin S | AP02243 | Bacillus brevis |
| Enterocin O16 | AP02520 | Enterococcus faecalis |
| Hc CATH | AP02569 | Venom gland, spleen and lung, annulated sea snake, Hydrophis cyanocinctus |
| GL13K | AP02570 | A synthetic peptide derived from human parotid secretory protein |
| Holothuroidin1 | AP02622 | sea-cucumber, Holothuria tubulosa |
| Holothuroidin2 | AP02623 | sea-cucumber, Holothuria tubulosa |
| Paracentrin 1 | AP02624 | Coelomocyte cytosol, the sea urchin, Paracentrotus lividus |
| ToAP1 | AP02758 | Tityus obscurus |
| ToAP2 | AP02759 | Tityus obscurus |
| Con10 | AP02761 | Opisthacanthus cayaporum |
| Horine | AP02856 | Artificial, designed based on temporin-SHf |
| H4 | AP02864 | Artificial, combined BMAP-27 and OP-145 |
| Phylloseptin-co | AP02867 | Phyllomedusa coelestis, South America |
| Esculentin 1A(1-21) | AP02872 | Natural fragment |
| SAAP-148 | AP02875 | Artificial, designed based on LL-37 |
| Dhvar 4 | AP02876 | Artificial |
| AMP17 | AP02894 | Housefly, Musca domestica |
| Dermaseptin-PH | AP02924 | Orange-legged leaf frog, Pithecopus (Phyllomedusa) hypochondrialis, South America |
| Hyicin 4244 | AP02925 | Staphylococcus hyicus 4244 |
| Polymyxin B | AP02928 | Bacillus aerosporus Greer |
| CATHPb1 | AP02964 | Burmese python (Python bivittatus) |
| Temporin-GHc | AP02969 | Skin, Hylarana guentheri, China, Asia |
| Temporin-GHd | AP02970 | Skin, Hylarana guentheri, China, Asia |
| Moronecidin like | AP03001 | Tiger tail seahorse, Hippocampus comes |
| Mastoparan-C | AP03041 | Venom, the European Hornet, Vespa crabro |
| Nigrosin HLM | AP03048 | Motif-targeted peptide design |
| VLL-28 | AP03049 | Sulfolobus islandicus |
| Phylloseptin-PHa | AP03057 | Skin secretions, Orange-legged leaf frog, Pithecopus hypochondrialis, South America |
| Japonisin 2LF | AP03059 | Skin secretion, Fujian Large-headed frog, Limnonectes fujianensis, China, Asia |
| ZmD32 | AP03072 | Corn, Zea mays |
| SA-CATH | AP03077 | Snake, Sinonatrix annularis, China, Asia |
| Cm-CATH2 | AP03079 | Lung, green sea turtle, Chelonia mydas |
| Hs02 | AP03104 | Predicted intragenic antimicrobial peptide, Homo sapiens |
| Dermaseptin-PT9 | AP03133 | Skin secretion, Phyllomedusa tarsius, purchased in Peru, South America |
| Brevinin-1GHa | AP03178 | Skin, Guenther's Frog, Sylvirana guentheri, Hylarana guentheri (old), Asia |
| Dermaseptin AC4 | AP03185 | Skin secretion, the red-eyed tree frog, Agalychnis callidryas, North America (Mexico) to Central America |
| Rpdef1alpha | AP03192 | manila clam, Ruditapes philippinarum |
| Kassiniatuerin-3 | AP03219 | Kassina senegalensis, Africa |
| Verine | AP03236 | database filtering+structure-based refinement |
| Phylloseptin-PTa | AP03239 | Skin secretions, the brown-bellied leaf frog, Phyllomedusa tarsius, South America |
| Brevinin-1H | AP03240 | Skin secretions, Hainan cascade-frog, Amolops hainanensis; China, Asia |
| Temporin-PF | AP03258 | Skin secretions, Pelophylax fukienensis, China, Asia |
| t-DPH1 | AP03307 | Skin secretion, the northern orange-legged leaf frog, tiger-legged monkey frog, Phyllomedusa hypochondrialis, South America |
| Astucin | AP03343 | Aspergillus tubingensis A01, isolated from soil |
| Kassporin-KS1 | AP03345 | Skin secretions, the African hyperoliid frog, Kassina senegalensis, Africa |
| Phibilin | AP03424 | Philomycus bilineatus |
| Nigrocin-PN | AP03425 | Pelophylax nigromaculatus, Asia |
| Temporin-1CEh | AP03428 | Skin Secretion, Chinese forest frog, Chinese brown frog, Rana chensinensis, China, Asia |
| Brevinin-GR2 | AP03443 | Hylarana guentheri, China, Asia |
| PN5 | AP03449 | Pine needles, Pinus densiflora Sieb. et Zucc |
Table 1: Antibiofilm peptides and their source.
Antibiotics vs. antibiofilm:
As discussed above antibiotic resistance is arising as a global crisis, Hancock et al., postulated that in USA resistant bacteria infects near about 2 million people per year with which mortality number is 23 000 including sepsis for which the main treatment is antibiotics, only the death rate rises to 210 000 and if we talk about worldwide it is 5 million[26]. Despite this contumacious behavior of biofilm and reoccurring infections, we were able to produce only a few antibiotics which can overcome this threat, although oritavancin a semisynthetic derivative of glycopeptide antibiotic chloroeremomycin is mostly active against gram-positive bacteria and not against gram-negative pathogens. Only fluoroquinolones which are approved more than 50 y ago, no new type of drug has been approved for gram-negative organisms[27]. Biofilms has a key role in severe infections because they can appear on any given body and can also cover implanted device. If bacteria have biofilm covering, it becomes 10 to 1000 times more resistant to conventional antibiotics treatment because biofilm impedes penetration better than planktonic counterparts which are studied clinically[28-31]. Unfortunately, none of the antibiotics that are now being prescribed by doctors have been specifically targeted to inhibit biofilms, as their development was focused on taking advantage of their capacity to kill planktonic bacteria. Even nowadays, antibiotic developers hardly ever use animal models for biofilm infection or evaluate the susceptibility of resistant biofilm occurrence[26,32]. Many theories have been given for antibiotic resistance developed by biofilms, but resistance due to biofilm is modulated through a plethora of molecular mechanisms and it has adaptive and it leads to the acquisition of inheritable resistance[29]. Some of them are discussed below.
Mechanism of action of peptides as antibiofilm:
According to the studies done by by Raheem N et al.[33], and Batoni, G et al., AMPs can perform various activities and one of the important effects of antibiofilm using one or more Mechanism of Action (MOAs). However, the specific mechanism and function usually depend upon the sequence of peptides. The first mechanism to exhibit antibiofilm property is membrane disruption where multiple functions are involved, the destruction of membrane potential and membrane permeabilization as the peptide will able to penetrate the biofilm layer. Second is cell signalling where two mostly observed and followed mechanisms quorum sensing and twitching motility is observed. In quorum sensing, AMPs are supposed to prevent the spread of infectious planktonic bacteria by regulating gene expression and controlling the population. EPS degradation is another approach followed by antibiofilm agents. Stringent response inhibition includes processes like amino acid starvation and iron starvation and other targets like the downregulation of genes forming biofilms was also shown by AMPs[33].
Application of AMPs:
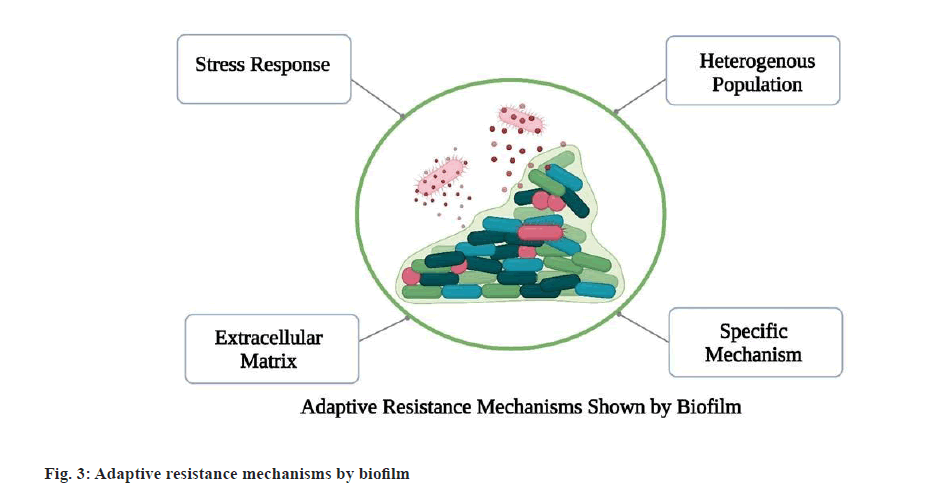
Antibacterial activity: A significant number of peptides possess broad-spectrum inhibitory antibacterial properties against vancomycin-resistant enterococci, MRSA and Listeria monocytogenes. As postulated by Li et al., Peptide 5 YIRKIRRFFKKLKKILKK-NH2 exhibited broad spectrum activity at Minimal Inhibitory Concentration (MIC) 8-16 µg/ml and Peptide 9 SYERKINRHFKTLKKNLKKK-NH2 at MIC 16-32 µg/ml against antibiotic-resistant strains of S. aureus with low cytotoxicity[34]. Fig. 3 shows how the biofilm produced by the bacteria resist to the antibiotics.
Antifungal activity: These peptides can counter the pathogenic fungus Aspergillus and Candida albicans,yeast infections showing enhanced multidrug resistance. As reported by Madanchi et al., synthetic peptide AurH1, having MIC of 7.3‐125 μg/ml showed promising results against Candida albicans as well all fungi strains inhibition with cytotoxicity killing 20 % human cells[35].
Antiviral peptides: Virus endangers humans by causing life-threatening infections. HIV and HCV can cause long-term lifetime harm to human life, recent Covid-19 outbreak has caused millions of lives to the death. Strong and promising antiviral drugs are of utmost need in this era. Peptides as potent antiviral agents can inhibit virus cell attachment and fusion with the host cell membrane, destroying the envelope and inhibiting replication[36] Anti-HIV peptides such as α and β Defensins, cathelicidin LL-37, gramicidin D, caerin 1, maximin 3, frog magainin 2, dermaseptin-s1 dermaseptin-s4 as well siamycin-I ,siamycin-II and RP71955 are most active and proved to be efficacious[37]. Enfuvirtide a viral fusion inhibitor peptide is also been commercialized for combination therapy for HIV as Fuzeon™.
Anticancer peptides: Cancer is indeed a most challenging disease spread across the globe causing millions of deaths. Yet research is continued to find promising curable drugs. Peptides show anticancer mechanisms by recruiting immune cells (such as dendritic cells) to kill tumor cells ultimately as immunomodulatory. Inducing cell death or apoptosis of cancer cells and interfering with gene transcription and translation of tumor cells by limiting protein functions. As reported by Arias et al., in vitro study of Tripticin against Jurkat T cell successfully showed toxicity with 18.3 μM, whereas indolicidin and puroindoline A can also be candidates for anticancer activity[38].
Commercial AMP’s:
A number of peptides have got approval from the FDA to be used as therapeutic agents as of their broad-spectrum activity, disease targeting and better mechanism of action than conventional antibiotics.
Bacitracin is a formulation composed of a cyclic peptide mixture targeting pneumonia and empyema in infants. Acting by creating interference in the cell wall and peptidoglycan layer synthesis. Fuzeon™ Enfuvirtide contains a small peptide. Used in the treatment of HIV-1. It usually acts by inhibiting of 6HB structure and blocking viral fusion. Oritavancin is a formulation consisting of glycopeptide. Used for acute bacterial skin and structural infection. It follows the cell membrane disruption mechanism. Telvancin formulation contains cyclic lipoglycopeptide in it which is useful in methicillin-resistant Staphylococcus aureus and other gram-positive infections acts by creating interference in the cell wall and peptidoglycan synthesis[39]. Table 2 contains the patents which are approved for use of AMPs as antibiofilm agents.
| S. No | Patent No | Patent Year | Characteristic | Reference |
|---|---|---|---|---|
| 1 | US9339525B2 | 2016 | Cysteamine antibiofilm agent and another antimicrobial peptide used for treatment of microbial infection | [45] |
| 2 | US9980497B2 | 2018 | Discussed about methods for how to use antimicrobial peptides possessing antibiofilm property in severe wound infections. Formulation contained chelating agent, zinc salt and antimicrobial peptide. | [46] |
| 3 | US20200109171A1 | 2021 | Peptide’s AMP principle is “Carpet model” for antimicrobial activity as well cationic property and amino acid sequencing and amphiphilic allows them to penetrate into bacterial cell and leading to death | [47] |
| 4 | US20220064219A1 | 2022 | Cationic antimicrobial peptides possess ability to activate innate immunity and good antimicrobial property and can kill both gram-negative and gram-positive bacteria and they prevent mutation | [48] |
| 5 | EP3743434A1 | 2022 | This immunomodulatory antibiofilm contains 7-14 amino acids which inhibits the bacterial biofilm growth, enhancing innate immunity and selective proinflammatory response | [49] |
Table 2: Patents of antimicrobial peptides having antibiofilm activity.
Nanocarriers for AMP:
The delivery system plays an important role in the pharmacodynamic effect of a drug. It also impacts pharmacokinetics. In the conventional delivery route, almost all the drugs get degraded. Parenteral shows maximum bioavailability of the drug in systemic circulation but it is not compliable to patient. It is a challenge for drugs to show effect without affecting the body for a non-invasive delivery system is necessary. Nanocarriers gained attention as they enable targeted and effective delivery of drugs with a minimum number of side effects. Nanocarriers have wide applications as they can be used for drug delivery and diagnosis. Most of the time effectiveness of AMP’s thwarted by their low solubility and their stability is poor as they get chemically degraded in the biological system.
As the study performed by Gontsarik et al.[40], the antibacterial activity of Cathelicidin LL-37 dispersed in Glycerol Mono-Oleate (GMO) based cubosome against Escherichia coli. Loaded micelles with GMO: LL-37 having a ratio of 50:50 have rapid elimination of bacteria and preferable stability. Studies have shown that bactericidal activity was improved compared to free-flowing LL-37.
Conclusion
One of the biggest challenges in medicine is finding new therapeutic approaches to combat infections caused by biofilms, using antibiotics for high concentrations can lead to cytotoxicity. As mentioned above, AMP possesses wide and broad-spectrum activity and microorganisms could not produce resistance against them. Using AMPs in combination with substances that can dissolve the biofilm matrix is a promising combinatorial strategy that can be anticipated. AMPs are becoming a global research hotspot globally, but stability and solubility remain underlying issues. Despite being considered as an alternative to antibiotics, there are still undiscovered antimicrobials and the poor pharmacokinetics of peptide drugs makes it difficult to use existing AMPs effectively.
Mutation does not affect AMPs. Bacteria cannot develop resistance easily against AMPs because they show activity across the entire covering of bacteria and also broadens the capability of the host immune system. AMP contains amino acids when metabolism takes place, the amino acids get separated and those can contribute to vital biosynthetic pathways undergoing in the body, thus adverse drug reaction occurrence will be limited. On the other hand, antibiotic renal clearance is not assured so they are susceptible to cause toxicity
Nano system is an effective delivery system for peptide and nucleic acid therapeutics and is effective against biofilm infections. Nanocarriers as a promising delivery system, implementing them for AMP delivery can facilitate them for clinical trial studies this can be a crucial step for their implementation in actual treatment. Further research has to be done for the stability and solubility of AMP’s. As they already have broad-spectrum activity and can act as a potent drug. AMPs are potential antiviral candidates, so they can also be used in a combinational therapy. Researchers must engage themselves in synthetic AMP development.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ruhal R, Kataria R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol Res 2021;251:126829.
[Crossref] [Google Scholar] [PubMed]
- Bu F, Liu M, Xie Z, Chen X, Li G, Wang X. Targeted anti-biofilm therapy: Dissecting targets in the biofilm life cycle. Pharmaceuticals 2022;15(10):1253.
[Crossref] [Google Scholar] [PubMed]
- Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat Rev Microbiol 2017;15(12):740-55.
[Crossref] [Google Scholar] [PubMed]
- Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, et al. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect Dis 2016;16(2):239-51.
[Crossref] [Google Scholar] [PubMed]
- Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: An emerging category of therapeutic agents. Front Cell Infect Microbiol 2016;6:235805.
[Crossref] [Google Scholar] [PubMed]
- Shahrour H, Ferrer-Espada R, Dandache I, Bárcena-Varela S, Sánchez-Gómez S, Chokr A, et al. AMPs as anti-biofilm agents for human therapy and prophylaxis. Adv Exp Med Biol 2019:257-79.
[Crossref] [Google Scholar] [PubMed]
- Lei J, Sun L, Huang S, Zhu C, Li P, He J, et al. The antimicrobial peptides and their potential clinical applications. Am J Transl Res 2019;11(7):3919.
[Google Scholar] [PubMed]
- Fadaka AO, Sibuyi NR, Madiehe AM, Meyer M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics 2021;13(11):1795.
[Crossref] [Google Scholar] [PubMed]
- Chawla M, Verma J, Gupta R, et al. Antibiotic potentiators against multidrug-resistant bacteria: Discovery, development, and clinical relevance. Front Microbiol 2022;13:887251.
[Crossref] [Google Scholar] [PubMed]
- Catalano A, Iacopetta D, Ceramella J, Scumaci D, Giuzio F, Saturnino C, et al. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022;27(3):616.
[Crossref] [Google Scholar] [PubMed]
- Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem 2009;78(1):119-46.
[Crossref] [Google Scholar] [PubMed]
- Paul D, Verma J, Banerjee A, Konar D, Das B. Antimicrobial resistance traits and resistance mechanisms in bacterial pathogens. Antimicrob Resistance 2022:1-27.
- Karygianni L, Ren Z, Koo H, Thurnheer T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 2020;28(8):668-81.
[Crossref] [Google Scholar] [PubMed]
- Yu M, Chua SL. Demolishing the great wall of biofilms in Gram‐negative bacteria: To disrupt or disperse? Med Res Rev 2020;40(3):1103-16.
[Crossref] [Google Scholar] [PubMed]
- Singh A, Amod A, Pandey P, Bose P, Pingali MS, Shivalkar S, et al. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed Mater 2022;17(2):022003.
[Crossref] [Google Scholar] [PubMed]
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: An emergent form of bacterial life. Nat Rev Microbiol 2016;14(9):563-75.
[Crossref] [Google Scholar] [PubMed]
- Schulze A, Mitterer F, Pombo JP, Schild S. Biofilms by bacterial human pathogens: Clinical relevance-development, composition and regulation-therapeutical strategies. Microb Cell 2021;8(2):28.
[Crossref] [Google Scholar] [PubMed]
- Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS suppl 2013;121:1-58.
[Crossref] [Google Scholar] [PubMed]
- Ruiz J, Calderon J, Rondón-Villarreal P, Torres R. Analysis of structure and hemolytic activity relationships of antimicrobial peptides (AMPs). Adv Computation Biol 2014:253-8.
- Ruijne F, Kuipers OP. Combinatorial biosynthesis for the generation of new-to-nature peptide antimicrobials. Biochem Soc Trans 2021;49(1):203-15.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez AA, Otero-González A, Ghattas M, Ständker L. Discovery, optimization, and clinical application of natural antimicrobial peptides. Biomedicines 2021;9(10):1381.
[Crossref] [Google Scholar] [PubMed]
- Münzker L, Oddo A, Hansen PR. Chemical synthesis of antimicrobial peptides. Antimicrobial Peptides: Methods and Protocols 2017:35-49.
[Crossref] [Google Scholar] [PubMed]
- Haney EF, Straus SK, Hancock RE. Reassessing the host defense peptide landscape. Front Chem 2019;7:435645.
[Crossref] [Google Scholar] [PubMed]
- Graf M, Wilson DN. Intracellular antimicrobial peptides targeting the protein synthesis machinery. Antimicrobial Peptides: Basics for Clinical Application 2019:73-89.
[Crossref] [Google Scholar] [PubMed]
- Shah P, Hsiao FS, Ho YH, Chen CS. The proteome targets of intracellular targeting antimicrobial peptides. Proteomics 2016;16(8):1225-37.
[Crossref] [Google Scholar] [PubMed]
- Hancock RE. Rethinking the antibiotic discovery paradigm. EBioMedicine 2015;2(7):629-30.
[Crossref] [Google Scholar] [PubMed]
- De La Fuente-Núñez C, Cardoso MH, de Souza Cândido E, Franco OL, Hancock RE. Synthetic antibiofilm peptides. Biochim Biophys Acta 2016;1858(5):1061-9.
[Crossref] [Google Scholar] [PubMed]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science 1999;284(5418):1318-22.
[Crossref] [Google Scholar] [PubMed]
- De la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock RE. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 2013;16(5):580-9.
[Crossref] [Google Scholar] [PubMed]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol 2004;2(2):95-108.
[Crossref] [Google Scholar] [PubMed]
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 2013;3(4):a010306.
[Crossref] [Google Scholar] [PubMed]
- Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N. Applying insights from biofilm biology to drug development—can a new approach be developed? Nat Rev Drug Discov 2013;12(10):791-808.
[Crossref] [Google Scholar] [PubMed]
- Raheem N, Straus SK. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front Microbiol 2019;10:2866.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhu C, Ren B, Yin X, Shim SH, Gao Y, et al. Two optimized antimicrobial peptides with therapeutic potential for clinical antibiotic-resistant Staphylococcus aureus. Eur J Med Chem 2019;183:111686.
[Crossref] [Google Scholar] [PubMed]
- Madanchi H, Khalaj V, Jang S, Shabani AA, Ebrahimi Kiasari R, Seyed Mousavi SJ, et al. AurH1: A new heptapeptide derived from Aurein1. 2 antimicrobial peptide with specific and exclusive fungicidal activity. J Pept Sci 2019;25(7):e3175.
[Crossref] [Google Scholar] [PubMed]
- Jung Y, Kong B, Moon S, Yu SH, Chung J, Ban C, et al. Envelope-deforming antiviral peptide derived from influenza virus M2 protein. Biochem Biophys Res Commun 2019;517(3):507-12.
[Crossref] [Google Scholar] [PubMed]
- Madanchi H, Shoushtari M, Kashani HH, Sardari S. Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes New Infect 2020;34:100627.
[Crossref] [Google Scholar] [PubMed]
- Arias M, Haney EF, Hilchie AL, Corcoran JA, Hyndman ME, Hancock RE, et al. Selective anticancer activity of synthetic peptides derived from the host defence peptide tritrpticin. Biochim Biophys Acta Biomembr 2020;1862(8):183228.
[Crossref] [Google Scholar] [PubMed]
- Gomes B, Augusto MT, Felício MR, Hollmann A, Franco OL, Gonçalves S, et al. Designing improved active peptides for therapeutic approaches against infectious diseases. Biotechnol Adv 2018;36(2):415-29.
[Crossref] [Google Scholar] [PubMed]
- Gontsarik M, Buhmann MT, Yaghmur A, Ren Q, Maniura-Weber K, Salentinig S. Antimicrobial peptide-driven colloidal transformations in liquid-crystalline nanocarriers. J Phys Chem Lett 2016;7(17):3482-6.
[Crossref] [Google Scholar] [PubMed]
- Blower RJ, Barksdale SM, van Hoek ML. Snake cathelicidin NA-CATH and smaller helical antimicrobial peptides are effective against Burkholderia thailandensis. PLoS Negl Trop Dis 2015;9(7):e0003862.
[Crossref] [Google Scholar] [PubMed]
- Tao R, Tong Z, Lin Y, Xue Y, Wang W, Kuang R, et al. Antimicrobial and antibiofilm activity of pleurocidin against cariogenic microorganisms. Peptides 2011;32(8):1748-54.
[Crossref] [Google Scholar] [PubMed]
- Morroni G, Simonetti O, Brenciani A, Brescini L, Kamysz W, Kamysz E, et al. In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med Microbiol Immunol 2019;208:877-83.
[Crossref] [Google Scholar] [PubMed]
- Wang G, Mishra B, Epand RF, Epand RM. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta Biomembr 2014;1838(9):2160-72.
[Crossref] [Google Scholar] [PubMed]
- O'neil D, Mercer D, Charrier C, inventors; NovaBiotics Ltd, assignee. Inhibition of biofilm organisms. United States patent US 9,339,525. 2016.
- Gawande PV, LoVetri K, Yakandawala N, Froelich G, Madhyastha S, inventors; Kane Biotech Inc, assignee. Antimicrobial-antibiofilm compositions and methods of use thereof. United States patent US 9,980,497. 2018.
- Gruber KA. Anti-microbial peptides. Google Patents 2021.
- de la Fuente-Nunez C, Torres MD, Franco OL, inventors; University of Pennsylvania Penn, assignee. Antimicrobial and antibiofilm peptides sequences with metal-binding motifs. United States patent application US 17/446,102. 2022.
- Hancock R, Haney E, Hilchie A, Tcherkasov A, Brito-sanchez Y, inventors; University of British Columbia, assignee. Cationic peptides with immunomodulatory and/or anti-biofilm activities. 2020.
 .
.