- *Corresponding Author:
- V. R. Yasam
Department of Pharmaceutics, JSS College of Pharmacy, JSS University, Udhagamandalam-643 001, India
E-mail: 57ramesh@gmail.com
| Date of Submission | 15 December 2016 |
| Date of Revision | 11 April 2017 |
| Date of Acceptance | 08 January 2018 |
| Indian J Pharm Sci 2018;80(2): 223-234 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Epilepsy is one of the most common neurological disorders, characterized by recurrent spontaneous seizures and a major health problem that affects around 1-2 % of the population worldwide. The treatment generally includes antiepileptic drug therapies. However, despite the development of various antiepileptic drugs, about a third of patients are resistant to current pharmacotherapies. Non-drug therapies are also expanding in an exciting and unprecedented way since in patients with drug resistance, non-drug therapies like surgeries; implantable devices and diet are playing a key role in improving the quality of patient’s life. In this review, the current available non-drug therapies ranging from preclinical to clinical for controlling seizures in epileptic patients with drug resistance have been highlighted. Initial results from these therapies are quite promising, but considerable development is required before these treatments earn a place in the standard clinical care.
Keywords
Non-drug therapies, diet therapy, devices, radiosurgery, epilepsy
Epilepsy is a devastating multi-causal chronic disease characterized by recurring, unprovoked seizures, affecting about 1-2 % of the population worldwide. Despite the discovery of new molecules and antiepileptic drug (AED) therapies over the years, about 1/3 of epileptic patients do not become seizure free and become resistant to AED treatment. In patients with drug resistance, non-drug therapies play a vital role. In this review, an overview of epilepsy and available non-drug therapies, such as surgery, radiosurgery, dietary treatment and devices has given. It neither covers diagnostic devices related to magnetic resonance imaging (MRI), electroencephalographic (EEG) or positron emission tomography scans, chemical delivery vehicles for drugs, gene therapy, nor is the review comprehensive.
The surgical treatment of epilepsy has seen much progress in past 15 y. Surgery in the treatment and management of refractory focal epilepsy is established, but it is much less frequently used in status epilepticus (SE) and other seizure types like atypical, typical and myoclonic. Rapid advances in surgical technology and neuroimaging are the driving forces behind these changes. Parallel understanding of developments in epilepsy neurobiology bring us to a time when many novel surgical treatments will be either in pre-clinical/clinical trial or soon to begin application to human epilepsy [1-3].
Diet therapy was reported centuries ago, Hippocrates first noted that fasting treated seizures [4]. Hundred years ago, Guelpa and Marie rediscovered and reported the benefit of fasting on seizure control [5]. The use of ketogenic diet (KD) in the treatment of seizures was first reported by Wilder [6]. In this first report on use of a KD, ≥50 % of patients at the Mayo Clinic had significant seizure control. By the end of 1930s, development of new AEDs detracted interest away from the KD. Once again during 1970s, a modification of this classical diet was introduced using medium chain triglycerides (MCT) as an alternative fat source [7,8]. Later Kossoff et al. [9] and Pfeifer and Thiele [10] developed modified Atkins diet (MAD) and low glycemic index treatment (LGIT), respectively.
The use of devices, brain stimulation and microbeambased therapies has gained much attention recently. Even though some of the devices like Vagus Nerve Stimulator (VNS, Cyberonics, Inc., Houston, TX, USA.), Responsive Neurostimulator (RNS®, NeuroPace, Inc., Mountain View, CA, USA) are successful, but still significant development and controlled preclinical and clinical trials are required to establish it in standard clinical treatment protocols. Therefore, broad range of strategies to stop seizures and need for newer therapies in epilepsy remains high [11].
Epidemiology
The prevalence of active epilepsy is 9-10 per 1000 persons in low income countries and 5-8 per 1000 persons in high income countries [12,13], where even higher rates have been reported in rural areas [14]. Difference in the prevalence rates is dependent on various risk factors and it also differs from one country to other. This might be attributed to the living conditions, inadequate prenatal care, antinatal care and various infections [15]. This was proved from a metaanalysis [16], showing that annual incidence is 82 per 100 000 persons with an interquartile range (IQR) of 28-240 in low-income and middle-income countries and 45 per 100 000 persons with an IQR of 30-67 in high-income countries. Even though many AEDs have been developed but still drug-resistant epilepsy and its associated increased risk of death and debilitating psychosocial consequences are still on.
Pathophysiology
A seizure results from transient abnormal synchronisation of neurons in the brain that disturbs normal patterns of neuronal communication and results in waxing and waning of electrical discharges in the EEG (electrographic seizure). During this process, a sudden imbalance occurs between the excitatory and inhibitory forces with in the network of cortical neurons. Based on the level of disruption and site of seizure origin, signs and symptoms will be varied largely [17-19].
The neuronal network in the brain is highly interconnected and it allows for coordination of different tasks and behaviours. Any disturbance in the regular neuronal electrical impulses leads to seizures and its related comorbidities such as learning disabilities, depression and autistic features. Advancement of technology in imaging, neurophysiology and video recording have improved our ability to identify the proper reason behind the seizures and also identify exact epileptic foci in the brain, to establish whether seizures are focal or generalised from the start, and to define their propagation patterns [20,21]. Based on these developments in the field, site specific drug targeting can be achieved by identifying the exact location of the neurons involved in the initiation, spread, or termination of seizures.
At present several animal models are available to study the potential therapeutic agents for the epilepsy treatment and to identify the multiple causes leading to epilepsy [22]. Emergence of highly advanced technologies in imaging and electrophysiology can unravel the neuronal activity and its alteration during and after the epilepsy in brain [23-25].
Non-drug therapies to treat epilepsy
Brain surgery is preferred in patients where drug treatment is not effective in reducing seizures and it helps in improving the patient condition. It is considered when the obvious cause for epilepsy is in brain, such as scar tissue. The seizure type and part of the brain where it begins determines whether surgery is needed.
The first and most common form of epileptic surgery is resection surgery, where the seizure causing area in the brain will be identified and surgically removed. The next considered surgery is hemispherectomy, where the connection between two hemispheres will be disconnected surgically. It is considered mostly in children, where the hemisphere is poorly functional and involves in initiation of seizures. Other form of surgeries includes occipital and parietal lobectomy, where the structural abnormalities in the lobes of the brain like an abnormal tumour, scar tissue or lesions will be identified and removed surgically.
Temporal lobectomy is the most common and successful type of surgery, which involves removal of a portion of temporal lobe of the brain. Frontal lobectomy is the removal of a part of frontal lobe but it has less success rate when compared with temporal lobectomy. Most patients need to continue seizure medication even after surgery but they may need to take less.
The other less common types of surgeries include corpus callosotomy and multiple subpial transections. Corpus callosotomy surgery involves disconnection of large fibre bundles connecting two sides of the brain, the corpus callosum. Multiple subpial transections surgery is done in patients with localized epileptogenic areas, which are impossible to remove safely. In such cases, transections will be made in cerebral cortex to interrupt and disconnect the seizures generating area with the neighbouring parts of the brain without affecting the functions performed by those areas in brain [26-33].
Thermal ablation
Thermal ablation is a minimally invasive type of laser surgery treatment and its potential has been recognised for decades [34-37]. Through a small nick scalp incision in skull a laser fibre will be guided towards the seizure source, entire procedure can be viewed by MRI. This helps to target seizure focal point with pinpoint accuracy. In this process, laser destroys the small well defined seizure focal point in brain tissue without damaging the surrounding tissue. On contrary, typical standard surgeries need a large cranial opening with extensive scalp incision more deeply and long treatment time leading to high risk of bleeding and infection. The laser ablation system was approved by FDA in 2010. In this new technology, treatment and recovery time is very fast, with very low chances of bleeding and infection. Preliminary findings were found to be positive in controlling seizures with effectiveness lasting from few months to at least one year without any seizure recurrence. Majority of the patients can be discharged on the following day of operation in this method, which is not possible in standard epileptic operations [38].
Devices
Devices are developed not to cure seizures but they may control the symptoms and provides a new hope and options in drug resistant epilepsy. Seizure medication helps over 60 % of population with seizures but most of the people suffer from seizures even after medication. In such cases, alerting devices are much more helpful (Table 1) [39-63].
| Device | System | References |
|---|---|---|
| Devices for brain stimulation | ||
| Intracranial systems | ||
| DBS | Implant device | 39 |
| RNS | Implant device | 40 |
| Extracranial systems | ||
| Focal cooling and uncaging | Implant device | 41 |
| RTM Stimulation | Implant device | 42 |
| TDC Stimulation | Implant device | 43 |
| TNS | Implant device | 44 |
| VNS | Implant device | 45 |
| Devices for epileptic alerts | ||
| Accelerometer | Wearable device | 46 |
| BrainGate™ Neural Interface System | Sensor implant device | 47 |
| Epdetect | Mobile-phone-based device | 48 |
| Epilert | Mobile-phone-based device | 49 |
| Medpage ST-2 | Wearable device | 50 |
| NeuroPort System | Software-based multichannel sensor device | 51 |
| SmartWatch Alert | Watch-based device | 52 |
| SeizAlert | Wearable device | 53 |
| Devices for surgery | ||
| Cyber knife | - | 54 |
| Functional MRI | - | 55 |
| Gamma knife | - | 56 |
| High-resolution brain SPECT | - | 57 |
| Magnetoencephalography | - | 58 |
| NIRS | - | 59 |
| SIGFRIED | - | 60 |
| Tractography and diffusion tensor imaging | - | 41 |
| Miscellaneous devices for overall epilepsy care | ||
| Protective Headwear’s | Wearable | 61 |
| Rehabilicare | Cortical stimulators and mapping systems |
62 |
| Safety Place Mat® | Working mats | 63 |
Table 1: Devices and their Applications for Management of Epilepsy
Seizure alerting devices play an important role in detecting seizures, alerting family members and care givers thus helps in controlling the seizure behaviour. These devices are designed to alert but not to prevent seizures and its consequences. Most of the devices are triggered depending on the repeated shaking movements as in tonic-clonic seizures and they are proved ineffective in some form of seizures like absent, focal where big movements in the body are absent. Currently three types of alerting devices are available in the market namely camera, mattress and watch devices. These devices also have their own limitations like inability to detect some type of seizures, not practical in people living alone and it do not alert other conditions like change in heart rate and breathing problems. For their success, they have to be proved scientifically and clinically in large number of patients for different type of seizures [11,64].
Investigational stage devices
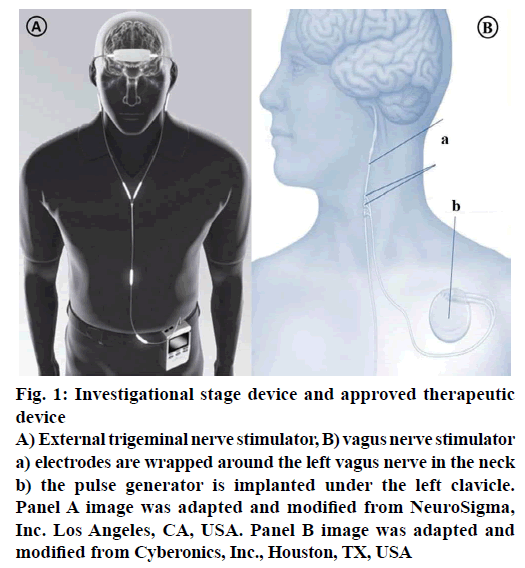
External trigeminal nerve stimulation (TNS) is an emerging and promising neuro-modulating noninvasive therapy for epilepsy. When compared with current therapies, it has its own unique advantages like lower potential of risk because there is no risk of placing electrode in the brain or risk of invasive devices; it can be delivered externally, bilaterally at reasonable costs. The treatment starts with a mild electrical stimulation of trigeminal nerve branches to modify the targeted brain regions activity. At present Neurosigma (Los Angeles, CA, USA) has the exclusive licensee for developing and manufacturing two embodiments of TNS (Figure 1): TNS with subcutaneous electrodes and implantable pulse generator (sTNS™) and external electrodes and an external pulse generator (eTNS™). Trigeminal nerve plays an important role in conveying the information to vagus nerve, locus coeruleus, cerebral cortex that is known to play a vital role in initiation and inhibition of seizures. It also helps in controlling the attention, mood and decision making ability of the patient by sending signals to the anterior cingulate cortex. Trigeminal nerve and its related structures stimulation have shown promising results in inhibiting seizures in animal models. At UCLA and USC, in 2011, a dual centre, randomised, active-controlled, double blind study on humans was studied. Currently phase-III is under planning stages [11,65]. Phase II, randomized, double-blind, active-control trial has shown positive results in patients. Subjects were randomised to control (eTNS, 2 Hz) and active treatment (eTNS, 120 Hz). Response rate was found to be ≥50 % in active treatment group and reduction in seizure frequency was also seen. During an 18 w acute treatment period, responder’s rate is 17.8 % by 6 w and 40.5 % by 18 w. Statistically at all times during these studies, treatment group showed a significant improvement compared with the control group proving TNS as a useful device and potential predictor of outcome for other forms of neurostimulation [64,65].
Figure 1: Investigational stage device and approved therapeutic device
A) External trigeminal nerve stimulator, B) vagus nerve stimulator a) electrodes are wrapped around the left vagus nerve in the neck b) the pulse generator is implanted under the left clavicle. Panel A image was adapted and modified from NeuroSigma, Inc. Los Angeles, CA, USA. Panel B image was adapted and modified from Cyberonics, Inc., Houston, TX, USA
Approved therapeutic devices
During 1950s, Penfield and Jasper demonstrated the ability of electrical stimulation to suppress epileptiform discharges on direct cortical stimulation in humans [66]. Nerve stimulation is done by a special device called vagus nerve stimulation (Figure 1), which also referred as “pacemaker for the brain”. It was approved for use in Europe in 1994 and by US FDA in 1997. It is an “open loop” device, meaning that there will not be any direct feedback to modulate therapy. This device is designed to control or prevent the seizures by sending mild electrical stimulations to the seizure causing areas of the brain via vagus nerve and controls the involuntary functions under it, such as heart rate thus helps in reducing the length and frequency of the seizures. These devices are ideal in patients not willing or not suitable for brain surgery and it is also considered in drug resistant epileptic patients. Vagus nerve stimulation was proved successful in reducing the seizures up to 30-40 and 10 % or fewer patients are rendered seizure-free. This device is round, flat, about a coin size. The surgical procedure includes implantation of the device under the upper chest skin and connecting its electrodes other end around the vagus nerve in the neck. Then the surgeon programmes the timing and strength of the impulses of the stimulator to a suitable level based on patient condition and need. Usually the stimulator will be set to give stimulations for 30 s in every 5 min during day and night. The stimulator device works on battery, which lasts for 6-10 y depending on the stimulation settings. With the patient under anaesthesia, the procedure takes 50-90 min and patient can be discharged later the same day depending on the patient condition. This device is not a substitute for seizure medications, patient can continue the medication while using this, however if it works, then the number or dose of medications can be reduced [11,64].
A European long-term, retrospective, open label, multicentre study was conducted in 347 children with drugresistant epilepsy (aged 6 mo to 17.9 y at the time of implant) for 24 mo. Data of change in seizure frequency of the predominant seizure type was collected, assessed and interpreted following VNS device implantation from baseline to 6, 12 and 24 mo. The results collected from baseline to 6, 12 and 24 mo showed 32.5, 37.6 and 43.8 % of patients respectively, had ≥50 % reduction in baseline seizure frequency of the predominant seizure type. The results demonstrated that vagus nerve stimulation therapy is a success as adjunct therapy in children with focal, genetic, structural epilepsies and predominantly generalised seizures caused by Lennox- Gastaut syndrome or Dravet syndrome and it was well tolerated over a 2 y follow-up period [67].
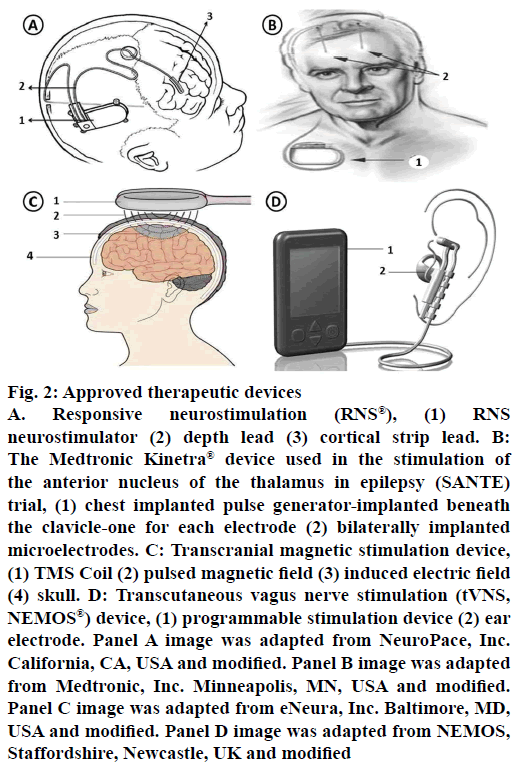
Responsive neurostimulation (RNS) is the first generation “closed loop” device (Figure 2). It will be implanted in skull with a strategy to directly stimulate subdural strips or electrodes on top or implanted in the brain near seizure foci to control or stop the seizures. In patients not responding to antiepileptic medication and patients with seizure foci in the brain which cannot be removed, in such cases, implanting this device is preferred. Once the device is set to particular EEG depending on the patients individual EEG and response, then this implant helps in detecting, collecting and recording the brain’s EEG. Whenever the implant identifies a seizure, it sends an electrical signal to control or disrupt the seizure activity. It is not an alternate to antiepileptic medication or cure for epilepsy but it helps in controlling or improving the patient condition. Depending on the patient response further modification in the therapy can be done for better results. A large multicentre trial was completed recently using RNS® system in a total 191 patients. After one month of implantation, patients were randomized (1:1) to either stimulation (treatment) or no-stimulation (sham) groups. Safety and efficacy were assessed during a blinded period (12 w) and open label period (84 w) during which all subjects received stimulation. By the end of 1 y, 44 % of patients achieved 50 % of reduction in seizure frequency; by 2 y it was 53 and 55 %, respectively. Overall, 9 patients have shown some side effects (one subdural hematoma, two intraparenchymal hemorrhages, three epidural hematomas and three seizure-related traumatic subdural hematomas) [11,64,68].
Figure 2: Approved therapeutic devices
A. Responsive neurostimulation (RNS®), (1) RNS neurostimulator (2) depth lead (3) cortical strip lead. B: The Medtronic Kinetra® device used in the stimulation of the anterior nucleus of the thalamus in epilepsy (SANTE) trial, (1) chest implanted pulse generator-implanted beneath the clavicle-one for each electrode (2) bilaterally implanted microelectrodes. C: Transcranial magnetic stimulation device, (1) TMS Coil (2) pulsed magnetic field (3) induced electric field (4) skull. D: Transcutaneous vagus nerve stimulation (tVNS, NEMOS®) device, (1) programmable stimulation device (2) ear electrode. Panel A image was adapted from NeuroPace, Inc. California, CA, USA and modified. Panel B image was adapted from Medtronic, Inc. Minneapolis, MN, USA and modified. Panel C image was adapted from eNeura, Inc. Baltimore, MD, USA and modified. Panel D image was adapted from NEMOS, Staffordshire, Newcastle, UK and modified
Other methods
The first therapeutic brain stimulation efforts were made by Delgado et al. (1952) and Heath (1963) in the field of psychiatry [69,70]. In 1979, a New York-based neurosurgeon, Irving Cooper carried out the first deep brain thalamic electrical stimulation for the treatment of epilepsy. The studies have shown positive results qualitatively by having an improvement in patient’s condition due to reduced seizure frequency. Recently Fisher and co-workers, performed stimulation of anterior nuclei (AN) of the thalamus with an open-loop device in a double blind, placebo controlled, randomised, multicentre trial called SANTE (Medtronic Kinetra® device, Minneapolis, MN, USA; Figure 2), studies. These studies have given promising results in controlling seizures and its frequency. In general, implantable pulse generators are separate; one under each clavicle but the device tested in SANTE contains two implantable pulse generators embedded in one device. Based on animal model results, AN was selected as a target for this trial. Stereotaxically the device was placed under clavicle and its two pulse generators for both the electrodes were placed one on each anterior thalamic nuclei. The device stimulates AN intermittently rather than continuously for producing effective results. It has shown favourable response in patients not benefitted from surgeries and medication. Complications with stimulation are occasional but more stimulated patients showed some symptoms like superficial infection, memory impairment, suicidal tendency, depression but on overall, they are within expected ranges further causing no harm. A total of 110 patients with bilateral convulsive seizure with tonic and/or clonic components and refractory partial seizures were randomized. For 3 mo of blinded phase, stimulation was given to only half (1:1) of the subjects, after 3 mo unblinded stimulation was followed. By 2 y, 54 % have ≥50 % reduction in seizure frequency increasing to 69 % by 5 y with 16 % of subjects completely free from seizures for at least 6 mo. By the end of study, 5 subjects died but none were symptomatic. As a result of the SANTE trial, Canada and European Union has approved anterior thalamic stimulation for the treatment of focal and evolving into a bilateral convulsive seizure with tonic and/or clonic components. Similar open-loop studies in humans on many other targets like subthalamic nucleus, centromedian nucleus are still in early stages and yet to be progressed for large scale trials [11,64,71].
Transcranial magnetic stimulation (TMS) is another method for brain stimulation with some benefits like focal treatment, non-invasive and considered as less painful, safe and direct. To date, however, only case reports and preliminary clinical trial data are available, some of them have showed disappointing results [72-75]. In this method, magnetic field induced brain currents were introduced with the help of magnetic stimulator coil from a safe distance to stimulate focally and deeply in the brain tissues (Figure 2). Based on the characteristics and advantages, figure-of-eight coils are widely used and preferred. The determinant factor for a successful TMS targeting depends on positioning of the magnetic stimulating coil over the seizure causing foci. Other factors of stimulation parameters like intensity, frequency, duration of exposure also affects the effectiveness of treatment. TMS device should be carefully used in patients on medication, pump or implants and untested stimulation parameters like heart diseases, brain lesions, pregnancy and seizure history should also be considered [11,38,64].
Transcutaneous vagus nerve stimulation (tVNS; Figure 2) is a non-invasive brain stimulation technique used to treat epilepsy by stimulating the left auricular branch of the vagus nerve at the ear conch. It is a newly developed tVNS (NEMOS®) device certified by CE (Cerbomed GmbH, Erlangen, Germany). It is an external device with a bipolar electrode attached to the skin of the left ear conch. To assess efficacy and safety of tVNS, a randomized, double-blind controlled trial (cMPsE02) was performed in patients with drugresistant epilepsy over a period of 20 w.
The study was to demonstrate and evaluate the safety, reduction in seizure frequency, sub-group analyses and superiority of add-on therapy with tVNS (stimulation frequency 25 Hz, n=39) versus active control (1 Hz, n=37) in reducing seizure frequency from baseline to end of treatment. By the end of the treatment, mean seizure reduction was 2.9 % in the 1 Hz group and 23.4 % in the 25 Hz group with mild adverse effects. Superiority of 25 Hz tVNS over 1 Hz tVNS could not be proven in this study but efficacy data revealed results that justify further trials with larger patient numbers with longer observation periods [76].
Radiosurgery
Radiosurgery is a promising alternative to respective surgery and drug resistant epilepsy. Even though it is a promising alternative but it has its own limitations. Modern radiosurgical clinical devices such as CyberKnife® and Gamma Knife® are successful up to some extent but often the safety of the normal tissues surrounding the lesion is at risk due to the radiosensitivity [77,78]. In such case, radiation dose has to be limited and should be delivered to the target area safely [79,80]. Third generation synchrotrons generates low energy photons (KeV) with an advantage of insignificant beam divergence, adequate dosing rate and spectrum of energy which helps in allowing the deposition of extremely steep lateral dose fall-off [81]. The precision with the synchrotron x-ray radiosurgery in animal experimental models of epilepsy helps in achieving better results, which is not possible with conventional mega voltage radiological devices used in clinics.
The microscopic range spatial fractionation of ionizing radiation was first used and reported in fifties but this is a failure due to inability of microbeam radiation to reach in deep brain tissues [82-85]. In murine brains, microplanar synchrotron of 50-150 KeV generated microbeams were investigated and it was found to produce sharply defined precise microbeam edges in the targeted tissues with high dose rate [86,87].
A novel use of microbeams was introduced recently by using a goniometer. In interlaced microbeam radiotherapy (IntMRT), brain will be rotated around a centre of rotation using goniometer by interlacing microbeams to deliver and deposit a high homogenous radiation dose precisely into distinct regions of brain, with no extensions on surrounding tissues; this is called IntMRT [88]. This irradiation allows a 200 times greater lateral dose fall-off than conventional radiotherapy. IntMRT was proved clinically significant and attractive in all pathologies that require submillimetric precision of targeting with neighbouring tissues preservation with confined inactivation, disconnection and/or destruction of small brain regions, even if close to vital or eloquent structures. At present more research and studies are continuing to apply IntMRT in mice and rat models of epilepsy and also in non-human primate models of epilepsy as proof of concept of its practicability in human epileptic patients. Present research on IntMRT is still active and promising enough for a realistic clinical transfer in the years to come [87].
Diet therapy
The KD is an alternative therapy to medications. Diet is strictly prescribed with high fat and low carbohydrates+proteins (3:1 or 4:1) ratio by weight, for a longer period of time. KD is also not suitable in some cases due to its restriction in metabolic diseases. In both the instances, diet will be supervised and modified continuously under the supervision of nutritionist and physician [89,90] based on the patients response towards the treatment [91].
But in many cases it was proved to be efficient than newer medication with more cost effectiveness. In almost all patients diet therapy has shown promising improvements in aggression and hyperactivity. Therefore it became a preferred treatment in patients with drug-resistant epilepsy and where surgery is not possible or where surgery cannot be performed due to various problems like affordability, facilities unavailability [92-96].
A number of possible mechanisms of action have been proposed to explain the KDs anticonvulsant actions. These hypothetical mechanisms are as follows: the caloric restriction hypothesis, the ketone hypothesis, the amino acid hypothesis, the brain lipids hypothesis, the metabolic hypotheses, noradrenaline hypothesis and the pH hypothesis [97-100].
In uncontrolled open-label prospective studies, children with intractable epilepsy when treated with KD, 7-15 % became seizure-free, 25-40 % has 90 % seizure reduction and 55 % have ≥50 % seizure reduction. KD was a success with favourable side effects profile in children but it’s been little studied in adults and major reason appears to be unpalatable diet [101]. In a study, over 15 adults were treated and evaluated for 12 mo with KD and MCT (n=2, n=11) or both (n=2, started on MCT and changed to KD because of gastro intestinal side effects). Ten of fifteen subjects stopped treatment due to lack of efficacy. Among 5 completers, 3 had <50 % and 2 had 50-90 % seizure reduction. In all studies and treatments, early stages of therapy should be monitored carefully due to associated adverse effects like weight loss, acidosis, drowsiness, gastro intestinal disturbances, nutritional deficiencies, increased prone to infections, pancreatitis and renal calculi. Based on the results after 4-6 mo of therapy, continuation or termination of treatment was considered on the suggestions of nutritionist and physician. In case of positive responders, treatment is continued up to 2-3 y and after gradually decreased [102].
Atkins diet is a modified traditional KD. Even though it has similarities with KD, it differs in many factors like no restriction on proteins, calories, fluids and only carbohydrates count. In this type of diet plan, patient can have food freely inside and outside the home and it can be started anywhere, anytime outside hospital. MAD is the modification of traditional Atkins diet with carbohydrate intake of 10-20 g/d along with good amount of fat intake [103]. Diet consists of approximately 1:1 (fat:(carbohydrate+protein)) weight ratio. It is highly efficient in both children and adults as it is less complex and more palatable compared to KD. Its efficacy in children with refractory epilepsy is similar to KD.
An observational, prospective, open-label study was conducted on the efficacy of the MAD in the very rare North Sea Progressive Myoclonus Epilepsy patients (aged 7-20 y). All the 4 patients enrolled (all are with known c.430G>T (p.Gly144Trp) mutation), were evaluated for the efficacy of the MAD for several clinical parameters at baseline and after 3 mo on the diet. By the end of the study, one patient has showed sustained improvement during long-term follow-up, where quality of life remained broadly unchanged in other three patients so they did not continue the diet. Nonetheless from the results it was confirmed that the MAD might be considered as a possible treatment in this devastating disorder.
Even an interventional, uncontrolled study was conducted in a Tertiary Care Pediatric Hospital in New Delhi from November 2012 to March 2014. Total 31 children were enrolled in the study, among them 21 children continued the treatment for 3 mo (8 children discontinued due to their refusal to eat and unsatisfactory seizure control) and 13 children up to 6 mo. The median age of the children enrolled was 18 mo (range 9-30 mo) at the start of the diet. From the results it was concluded that >50 % seizure reduction was observed in 17 (54.8 %) children at 3 mo and 9 (29 %) children at 6 mo [104].
In another prospective, open-label, uncontrolled study, 30 adults were evaluated for 6 mo, followed by openended extension. 53 % of subjects stopped treatment before 6 mo due to lack of efficacy and restrictiveness. Other subjects continued diet and completed 6 mo study. Among them, 33 % subjects achieved >50 % reduction in seizures, 13 >75 % seizure reduction and 3 % became seizure free. In all studies, neurologist must be involved before starting the diet plan. Supervision of dietician and physician is not that important to start a traditional Atkins diet but it is compulsory to consult and start the MAD under the supervision of them. Periodically, diet plan should be tracked and changes in height, weight, calorie intake and problems associated should be noted. Blood and urine should be monitored every 3 mo and monitoring of urine ketones frequently once or twice in a week is needed. Diet associated side effects are seen in some cases like loss of weight, increase in cholesterol, resultant ketosis. If the diet is not working out after 3-4 mo, then the diet plan should be stopped. If the diet gives positive results and patient becomes seizure free for 2-3 y, then the diet can be terminated successfully [90,91]. In 2005, LGIT was developed as an alternate to KD [10]. It can be considered when a KD centre is not available, if there is an extended wait time for KD initiation or KD is not tolerated. In a study of 11 subjects, 8 subjects achieved >50 % reduction in seizure frequency and 4 of these became completely seizure free. LGIT is a flexible therapy monitors daily total carbohydrate consumption and focuses on carbohydrates with low glycemic index. LGIT can be initiated as an outpatient, followed by dietician suggestions, monitoring person’s growth history and current diet intake. For further lowering glycemic index, carbohydrate diet can be consumed together with protein and fats. Patients should be monitored every 3 mo for blood tests, height and weight. Based on the studies reported, LGIT can be considered and recommended as a possible alternative to the classic KD. If seizure freedom is achieved, based on patient condition and physician suggestions the diet can be ended [105].
Software
Software is a platform for identifying, alerting, analysing, evaluating and comparing data obtained from patient. Software works by analysing userdefined seizure events over a given time period and all the events will be logged in a database for creating customizable reports and graphs of aggregate seizure data. Software tools ranging from emergency mobile phone alerts to high-tech seizure pattern analysis tools are presently available (Table 2).
| Software | Development stage |
|---|---|
| EpiTrax | In market |
| eemagine EEG | In market |
| Epivista | In market |
| Epilexia | In market |
| iPlan® Net | In market |
| IdentEvent™ | In market |
| Leonardo Brainmap | In market |
| Neuroport Software System | In market |
| NeuroScore | In market |
| NeuroGuide Deluxe QEEG 2.5.5 | In market |
| Net Station 4.3 | In market |
| NNAAF | Under research |
| PEAT | In market |
| RecogniZ’e | In market |
| SPM 8 | In market |
| TWin® | In market |
Table 2: Software to Support Epilepsy Care, their Development Stages
Diet-based therapy offers a safe, alternative, effective, proven cost effective treatment for patients. KD is extremely successful in number of one randomised trials and observational studies. An increasing research and literature on newer therapies like AD and LGIT indicates that these newer therapies also have a key role in seizure treatment. At present, many diet options and variations are available to make the diets more palatable and easier to administer with the help of a nutritionist and a neurologist. Future research and randomised controlled studies will help to define the timing of implementation, utility and their routine use in the range of treatments available for children and adults.
Devices are successful up to some extent only, fewer patients are benefitted and others are still going for other options like radiosurgeries. These surgical methods are useful in patients with focal initiated seizures and acting as a most reliable and accurate alternate when compared with others but this treatment is restricted and it is dependent on various conditions of patients. So it cannot be implied in large number of patients. Even though present available devices and surgeries are serving in various groups of patients successfully but they are not handy and flourished in all epileptic types. To outreach it into much more patients, extensive research is required in all drug and non-drug therapies.
In some cases, patients are looking for alternative form of therapies. Advances in neuroimaging, stimulation technology and radiosurgery development have expanded the pool of options for patients suffering from SE. Through multiscale, multi-disciplinary research and collaboration, implantable devices hold promising and exciting possibilities for epilepsy network diagnosis, mapping and improving epilepsy therapy. Emergence of different devices and targets of stimulation is a rapidly evolving field and will play a key role in management of SE. VNS played an important role for many years in the management of seizures. In well-designed clinical trials, RNS® and thalamic stimulation therapies have shown tolerability, with good efficacy. These therapies are slightly superior but the potential for serious complications is much higher to that of VNS. External TNS therapy seems to be promising and also a potential predictor of outcome. Other modalities of neurostimulations like TMS and tVNS are in advanced stages of investigation. Neurostimulation is a rapidly expanding field with the need of much more research and it is a promising field of hope for epilepsy patients. All the results are encouraging and need further intensive research and investigations to make solid evidence-based decisions. The next wave of therapies to develop will likely apply advances in neurobiology and treatment of SE.
Acknowledgments
The authors would like to dedicate this work to the memory of Dr. A. P. J. Abdul Kalam.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Logroscino G, Hessdorffer DC, Cascino G, Annegers JF, Hauser WA. Time trends in incidence, mortality, and case-fatality after first episode of status epilepticus. Epilepsia 2001;42:1031-35.
- Claassen J, Lokin JK, Fitzsimmons BF, Mendelsohn FA, Mayer SA. Predictors of functional disability and mortality after status epilepticus. Neurology 2002;58:139-42.
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998;338:970-76.
- Wheless JW. History of the ketogenic diet. Epilepsia 2008;49:3-5.
- Guelpa G, Marie A. La lutte contre l’epilepsie par la desintoxication et par la reducation alimentaire. Rev Ther Medico-Chirugicale 1911;78:8-13.
- Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clinic Proc 1921;2:307-8.
- Huttenlocher PR. Ketonaemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res 1976;10:536-40.
- Huttenlocher PR, Wilbourne AJ, Sigmore JM. Medium chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971;1:1097-103.
- Kossoff EH, Krauss GL, McGrogan JR, Freeman, JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology 2003;61:1789-91.
- Pfeifer HH, Thiele EA. Low-glycaemic index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005;65:1810-12.
- Stacey WC, Litt B. Technology insight: neuroengineering and epilepsy-designing devices for seizure control. Nat Clin Pract Neurol 2008;4:190-201.
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011;52:2-26.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology 2007;68:326-37.
- Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010;51:883-90.
- Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet 2012;380:1193-201.
- Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology 2011;77:1005-12.
- Bertram EH. Neuronal circuits in epilepsy: do they matter? Exp Neurol 2013;244:67-74.
- Avanzini G, Manganotti P, Meletti S, Moshé SL, Panzica F, Wolf P, et al. The system epilepsies: a pathophysiological hypothesis. Epilepsia 2012;53:771-78.
- Galanopoulou AS, Moshé SL. Neuronal network mechanisms-sex and development. In: Faingold C, Blumenthal H, editors. Neuronal Networks in Brain Function, CNS Disorders, and Therapeutics. Amsterdam: Elsevier; 2013.
- Schevon CA, Weiss SA, McKhann G Jr, Goodman RR, Yuste R, Emerson RG, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun 2012;3:1060.
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci 2013;33:11100-15.
- Löscher W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem Res 2017;42(7):1873-88.
- Ono T, Galanopoulou AS. Epilepsy and epileptic syndrome. Adv Exp Med Biol 2012;724:99-113.
- Coppola A, Moshé SL. Animal models. Handb Clin Neurol 2012;107:63-98.
- Engel J Jr, Pitkänen A, Loeb JA, Dudek FE, Bertram EH 3rd, Cole AJ, et al. Epilepsy biomarkers. Epilepsia 2013;54:61-69.
- http://www.webmd.com. [cited 2016 Dec 02 ]. Available from: https://www.webmd.com/epilepsy/guide/corpus-callosotomy#1.
- http://www.webmd.com. [cited 2016 Dec 02 ] Available from: https://www.webmd.com/epilepsy/guide/multiple-subpial-transection-mst#1.
- Vendrame M, Loddenkemper T. Surgical treatment of refractory status epilepticus in children: candidate selection and outcome. Semin Pediatr Neurol 2010;17:182-9.
- Mathew CW, Robin SBW. New experimental therapies for status epilepticus in preclinical development. Epilepsy Behav 2015:49:290-3.
- Bhatia S, Ahmad F, Miller I, Ragheb J, Morrison G, Jayakar P, Duchowny M. Surgical treatment of refractory status epilepticus in children: clinical article. J Neurosurg Pediatr 2013;12:360-66.
- Barros P, Brito H, Ferreira PC, Ramalheira J, Lopes J, Rangel R, et al. Resective surgery in the treatment of super-refractory partial status epilepticus secondary to NMDAR antibody encephalitis. Eur J Paediatr Neurol 2014;18:449-52.
- Kumar RM, Koh S, Knupp K, Handler MH, O'Neill BR. Surgery for infants with catastrophic epilepsy: an analysis of complications and efficacy. Childs Nerv Syst 2015;31:1479-91.
- Cuddapah VA, Thompson M, Blount J, Li R, Guleria S, Goyal M. Hemispherectomy for hemimegalencephaly due to tuberous sclerosis and a review of the literature. Pediatr Neurol 2015;53:452-55.
- Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942;26:179-3.
- Fry WJ, Mosberg W, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg 1954;11:471-8.
- Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron 1960;ME-7:166-81.
- Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: physical factors. J Physiol 1962;160:494-512.
- Ferenc AJ, Nathan M. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging 2008;27:391-9.
- http://www.medtronic.com. [Cited 2016 Dec 02]. Available from: https://www.medtronic.com/us-en/healthcare-professionals/therapies-procedures/neurological/deep-brain-stimulation.html.
- http://www.neuropace.com. [Cited 2016 Dec 02]. Available from: http://www.neuropace.com/the-rns-system/.
- Schachter SC, Guttag J, Schiff SJ, Schomer DL. Summit Contributors. Advances in the application of technology to epilepsy: the CIMIT/NIO Epilepsy Innovation Summit. Epilepsy Behav 2009;16:3-46.
- http://www.magstim-us.com. [Cited 2016 Dec 02]. Available from: https://www.magstim.com/products/transcranial-magnetic-stimulators.
- http://www.magstim-us.com. [Cited 2016 Dec 02]. Available from: https://www.magstim.com/products/tdcs-stimulators-and-accessories.
- DeGiorgio CM, Murray D, Markovic D, Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology 2009;72:936-38.
- http://www.cyberonics.com. [Cited 2016 Dec 02]. Available from: https://us.livanova.cyberonics.com/.
- ImpediGuide Ltd. Israel [Cited 2016 Dec 02]. Available from: https:// http://www.impediguide.com.
- http://www.cyberkineticsinc.com. [Cited 2016 Dec 02]. Available from: https://www.braingate.com/.
- http://www.epdetect.com. [Cited 2016 Dec 02]. Available from: http://www.epdetect.com/epilepsy_mobile_phone_application_features.html.
- http://www.biolertsys.com. [Cited 2016 Dec 02]. Available from: https://bio-lert.com/
- http://www.medpage-ltd.com. [Cited 2016 Dec 02]. Available from: https://www.medpage-ltd.com/catalogsearch/result/?q=Medpage+ST-2.
- http://www.blackrockmicro.com. [Cited 2016 Dec 02]. Available from: http://blackrockmicro.com/neuroscience-research-products/neural-data-acquisition-systems/neuroport-daq-system/.
- https://smart-monitor.com. [Cited 2016 Dec 02]. Available from: https://smart-monitor.com/about-smartwatch-inspyre-by-smart-monitor/.
- http://www.csiir.ornl.gov. [Cited 2016 Dec 02]. Available from: https://www.ornl.gov/news/three-ornl-technologies-honored-southeast-tech-transfer-group.
- https://stanfordhealthcare.org. [Cited 2016 Dec 02]. Available from: https://stanfordhealthcare.org/medical-treatments/c/cyberknife.html.
- http://www.kappametrics.com. [Cited 2016 Dec 02]. Available from: http://neoventus.com/clients/kappametrics/.
- Chen ZF, Kamiryo T, Henson SL, Yamamoto H, Bertram EH, Schottler F, et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg 2001;94:270-80.
- http://www.neurologica.com. [Cited 2016 Dec 02]. Available from: http://www.neurologica.com/portable-ct.
- http://www.vsmmedtech.com. [Cited 2016 Dec 02]. Available from: https://www.biospace.com/article/releases/vsm-medtech-ltd-awarded-whole-head-meg-contract-by-b-university-of-tubingen-b-/.
- http://nirsoptix.com. [Cited 2016 Dec 02]. Available from: https://www.nirsoptix.com/what-is-nirs.php.
- Brunner P, Ritaccio AL, Lynch TM, Emrich JF, Wilson JA, Williams JC, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav 2009;15:278-6.
- http://www.plument.com. [Cited 2016 Dec 02]. Available from: http://plument.com/protectacap.htm.
- http://www.integra-ls.com. [Cited 2016 Dec 02]. Available from: https://www.integralife.com/neurocritical-care/category/neurocritical-care.
- http://www.seizuresupport.com. [Cited 2016 Dec 02]. Available from: http://www.seizuresupport.com/products.htm.
- Jorge JA. Epilepsy: New Drug Targets and Neurostimulation. Neurol Clin 2013;31:785-98.
- DeGiorgio C, Soss J, Cook I, Markovic D, Gornbein J, Murray D, et al. Phase II double blind randomized controlled trial of trigeminal nerve stimulation in 50 subjects with drug resistant epilepsy. Neurology 2013;80:786-91.
- Penfield W, Jasper H. Electrocorticography. In: Penfield W, Jasper H, editors. Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown and Company 1954;692-738.
- Iren Orosz, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, et al. Vagus nerve stimulation for drug-resistant epilepsy: A European long-term study up to 24 months in 347 children. Epilepsia 2014;55:1576-84.
- Morrell MJ. RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295-304.
- Delgado JM, Hamlin H, Chapman WP. Technique of intracranial electrode implacement for recording and stimulation and its possible therapeutic value in psychotic patients. Confin Neurol 1952;12:315-19.
- Heath RG. Electrical self-stimulation of the brain in man. Am J Psychiatry 1963;120:571-7.
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment refractory epilepsy. Epilepsia 2010;51:899-908.
- Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol 2006;60:447-55.
- Joo EY, Hans SJ, Chung SH, Cho JW, Seo DW, Hong SB. Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clin Neurophysiol 2007;118:702-08.
- Theodore WH, Hunter K, Chen R, Vega-Bermudez F, Boroojerdi B, Reeves-Tyer P, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology 2002;59:560-62.
- Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ Jr, Pascual-Leone A, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav 2007;10:521-8.
- Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Grafet W, et al. Transcutaneous Vagus Nerve Stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimul 2016;9:356-63.
- Regis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schröttner O, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicentre study. Epilepsia 2004;45:504-15.
- Romanelli P, Anschel DJ. Radiosurgery for epilepsy. Lancet Neurol 2006;5:613-20.
- Sims E, Doughty D, Macaulay E, Royle N, Wraith C, Darlison R, et al. Stereotactically delivered cranial radiation therapy: a ten-year experience of linacbased radiosurgery in the UK. Clin Oncol R Coll Radiol 1999;11:303-20.
- St George EJ, Kudhail J, Perks J, Plowman PN. Acute symptoms after gamma knife radiosurgery. J Neurosurg 2002;97:631-4.
- Romanelli P, Bravin A. Synchrotron-generated microbeam radiosurgery: a novel experimental approach to modulate brain function. Neurol Res 2011;33:825-31.
- Zeman W, Curtis HJ, Gebhard KL, Haymaker W. Tolerance of mouse brain tissue to high energy deuterons. Science 1959;130:1760-1.
- Curtis HJ. The interpretation of microbeam experiments for manned space flight. Radiat Res Suppl 1967;7:258-64.
- Curtis HJ. The use of deuteron microbeam for simulating the biological effects of heavy cosmic-ray particles. Radiat Res Suppl 1967;7:250-7.
- Zeman W, Curtis HJ, Baker CP. Histopathologic effect of high-energy-particle microbeams on the visual cortex of the mouse brain. Radiat Res 1961;15:496-514.
- Anschel DJ, Bravin A, Romanelli P. Microbeam radiosurgery using synchrotron-generated submillimetric beams: a new tool for the treatment of brain disorders. Neurosurg Rev 2011;34:133-42.
- Studer F, Serduc R, Pouyatos B, Chabrol T, Bräuer-Krisch E, Donzelli M, et al. Synchrotron X-ray microbeams: A promising tool for drug-resistant epilepsy treatment. Phys Med 2015:31:607-14.
- Serduc R, Br€auer-Krisch E, Siegbahn EA, Bouchet A, Pouyatos B, Carron R, et al. High-precision radiosurgical dose delivery by interlaced microbeam arrays of highflux low-energy synchrotron X-rays. PLoS One 2010;5:e9028.
- Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia 2007;48:31-42.
- Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia 2009;50:304-17.
- Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev 2016;9: CD001903.
- Klein P, Tyrlikova I, Mathews GC. Dietary treatment in adults with refractory epilepsy: a review. Neurology 2014;83:1978-85.
- Selter JH, Turner Z, Doerrer SC, Kossoff EH. Dietary and medication adjustments to improve seizure control in patients treated with the ketogenic diet. J Child Neurol 2015;30:53-7.
- Vining EP. Long-term health consequences of epilepsy diet treatments. Epilepsia 2008;49:27-9.
- Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia 2004;45:1116-23.
- Kwiterovich PO Jr, Vining EP, Pyzik P, Skolasky R Jr, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 2003;290:912-20.
- Nylen K, Likhodii S, Burnham WM. The ketogenic diet: Proposed mechanisms of action. Neurotherapeutics 2009;6:402-05.
- Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. In: Noebels JL, Avoli M, Rogawski MA, et al., editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th ed. Bethesda (MD): National Center for Biotechnology Information (US); 2012.
- Statstrom CE, Rho JM. Epilepsy and Ketogenic Diet. 1st ed. Totowa (NJ): Humana Press 2004.
- Hartman AL, Gasior M, Vining EPG, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol 2007;36:281-92.
- Neal EG, Chaffe HM, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 2008;7:500-6.
- Lambrechts DA, Wielders LH, Aldenkamp AP, Kessels FG, de Kinderen RJ, Majoie MJ. The ketogenic diet as a treatment option in adults with chronic refractory epilepsy: efficacy and tolerability in clinical practice. Epilepsy Behav 2012;23:310-14.
- Atkins RC. Dr. Atkins’ New Diet Revolution. New York: Harper Collins; 2002.
- Mehta R, Goel S, Sharma S, Jain P, Mukherjee SB, Aneja S. Efficacy and tolerability of the modified Atkins diet in young children with refractory epilepsy: Indian experience. Ann Indian Acad Neurol 2016;19(4):523-27.
- Heidi HP, Elizabeth AT. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005;65:1810-12.