- *Corresponding Author:
- G. ChandraVadivelu
Research lab, GIET School of Pharmacy, Rajahmundry, Andhra Pradesh 533296, India
E-mail: gopi@giet.ac.in
| Date of Received | 19 February 2022 |
| Date of Revision | 07 April 2023 |
| Date of Acceptance | 05 March 2024 |
| Indian J Pharm Sci 2024;86(2):381-391 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A prodrug is a chemically inert drug precursor, which upon biotransformation liberates the pharmacologically active parent compound. It is also called proagent, latentiated drug, bio reversible derivative and congeners. There are several reasons to utilize prodrug strategy in drug design. The drug is insufficient in solubility, absorption, distribution, lack of target site-specificity, prolonged-release, toxicity, instability, poor acceptance and formulation issues can be made to prodrug. The concept of prodrug acts as a significant alternative to solve pharmaceutical and pharmacokinetic-related problems of drug molecules. Though a few studies have been concerned with some aspects of prodrugs, surprisingly there is no review on the complete information about prodrug and their recent applications. In the present study, an attempt had been made on the design, synthesis, development, bioactive pathway and latest research findings of newly synthesized prodrugs and their therapeutic uses.
Keywords
Drug design, prodrug, synthesis and development, bioactive pathway, recent therapeutic applications
The word Prodrug was introduced by Adrien Albert in 1951 and the concept was identified by Harper in 1959[1,2]. According to Harper, the word latentiated indicating to drugs that need bioactivation[3]. This description is the most suitable even at present moment and is consistent with the International Union of Pure and Applied Chemistry (IUPAC) definition which states that a prodrug is a biologically inactive molecule that is altered into an energetic active drug by an enzymatic or chemical process[4]. It is also called a bioreversible derivative, latentiated drug, pro-agent and congeners etc.,[5-7]. Many enzymes involving the activation of prodrugs are oxidoreductases like DT-diaphorase, β-glucuronidase, carboxylesterase and Cytochrome P450 (CYP450)[8-10]. Pharmaceutical scientists are often facing serious formulation problems including inadequate oral absorption, poor solubility, instability, rapid metabolism, short half-life and toxicity[11-13]. These problems lead to the growth of a comparatively great number of prodrugs. The healing power of the prodrug is enhanced by not only minimizing the unacceptable and harmful properties but also by maintaining the high selectivity on the targeted site[14,15]. In addition to that several prodrugs have gained massive experimental success in clinical studies. Therefore, the prodrug approach is quickly becoming a fundamental part of the drug discovery process. At present, 5 %-8 % of the therapeutic agents are categorized as prodrugs, and around 15 % of all new drug compounds are synthesized each year as prodrugs[16]. Prodrugs have been utilized in a broad diversity of therapeutic areas including antiulcer[17], analgesic[18], anti-hypertension[19], anti-influenza[20], anti-biotics[21], anticoagulant[22], antifungal[23], anticancer[24], anti-inflammatory[25] and anaesthetic agents[26].
In recent years, many scientists were prepared different prodrug molecules through carrierlinked and chemical modification[27,28]. But, there is no review concerning the complete information including the recent progress of prodrugs and their therapeutic activities. Therefore, the present work has been targeting a comprehensive summary of design, synthesis, development, bioactive pathway and recent therapeutic applications.
Preparation and Development of Prodrugs
Reason for prodrugs:
There are several reasons why one may wish to develop a prodrug strategy in drug design. Drugs having poor solubility, inappropriate absorption and improper distribution, lack of target sitespecificity, prolonged release, reduced toxicity, instability, poor acceptance and formulation issues can be made to prodrug[29-31].
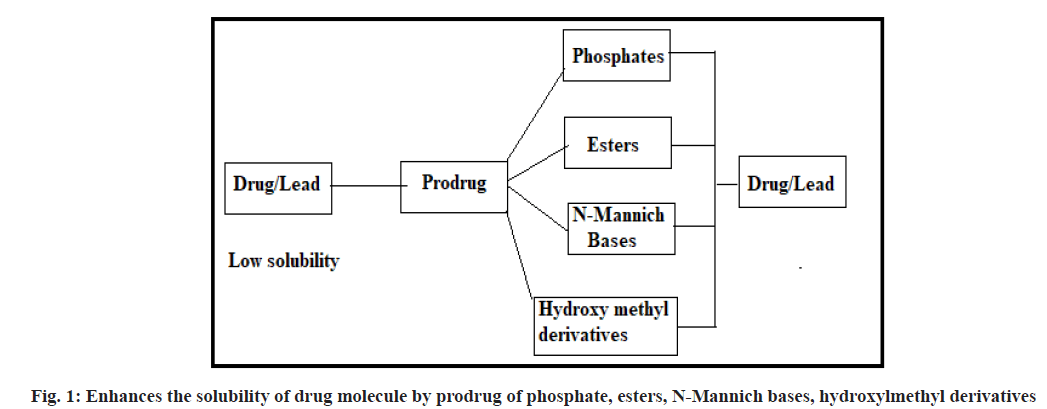
Enhances the solubility:
Poor solubility and low dissolution rate of poorly water-soluble drugs in the aqueous gastrointestinal fluids often cause insufficient bioavailability. The major challenge with the design of oral dosage forms lies in their poor bioavailability. Consider an active drug that is insufficiently soluble in water, so that it cannot be injected in a small dose. The solubility of active drug molecules is enhanced by converting them into prodrugs of phosphate, ester, N-Mannich base and hydroxymethyl groups etc., a water-soluble group could be attached which could be metabolically released after drug administration. For example, methyldopa is a zwitter ion and although charged, its crystal structure causes it to be only poorly water-soluble. To formulate this drug for injection, it is converted into ethyl ester which may be formulated as hydrochloride salt (fig. 1).
Improve the absorption and distribution:
If the drug is not absorbed and transported to the target site in sufficient concentration it can be made more water-soluble or lipid soluble, depending on the desired site of action. Once absorption occurs or when the drug is at the appropriate site of action, the water or lipid soluble group is removed enzymatically. The low absorption of antiviral acyclovir is increased by orally active prodrug valacyclovir, the L-valyl ester of acyclovir which is rapidly hydrolysed by first-pass intestinal metabolism. The prodrug is converted into Acyclovir in humans by the hydrolase enzyme present in the liver and gut.
Improve the target site-specificity:
Specificity for a particular organ or tissue can be made, if there is a high concentration of uniqueness of enzymes, present at that site which can cleave the appropriate appendages from the prodrug and unmask the drug. Gamma Aminobutyric Acid (GABA) is too polar, to cross the bloodbrain barrier, its effective lipophilic analogue of GABA (probabide) that crosses the blood-brain barrier, releases GABA inside the brain and shows anticonvulsant activity. In this example, the lipophilicity was increased, so that they could diffuse through the membranes.
Prolonged-release:
It may be desirable to have a steady low concentration of a drug released over a long period. The drug may be altered, so that it is metabolically converted to the active form slowly. For example, fluphenazine has a shorter duration of action (6-8 h), but prodrugs fluphenazine decanoate has duration of activity of about a month.
Reduces the toxicity:
A drug may be toxicity in its active form and would have a greater therapeutic index if it were administered in a nontoxic, inactive form that can be converted to the active form only at the site of action. Epinephrine is a drug used in the treatment of glaucoma. The drug has only limited local use because of poor penetration. But higher doses of it irritate the eyes and have undesirable cardiovascular effects. The ester prodrug dipivaloloyl epinephrine increases lipophilicity, which has better intraocular penetration and thus side effects are limited.
Improve the stability of the drug:
Many drugs are unstable and may either break down on prolonged storage or are degraded rapidly on administration. Several drugs may decompose in gastrointestinal tract when used orally or metabolized and rendered inactive, before when it reaches the site of action. Although enteric coatings may be used, it is also possible to utilize prodrug design to overcome this problem. The structure may be modified to block that metabolism until the drug is at the desired site. An antineoplastic drug azacytidine hydrolyzes readily in acidic pH, but the bisulphite prodrug is more stable.
Improve the patient acceptance:
An active drug may have an unpleasant taste or odour, produces gastric irritation, or causes pain when administered. The structure of the drug can be modified to alleviate these problems but once administered, the altered drug can be metabolized into the active drug. Several drugs (nicotinic acid, salicylic acid, kanamycin and diethylstilbosterol) cause irritation and damage to the gastric mucosa. The prodrug of the above-mentioned therapeutic agents overcomes such problems of gastric distress.
Solving the formulation problems:
If the drug is a volatile liquid, it would be more desirable to prepare it in a solid form, so that it could be formulated as a tablet. An inactive solid derivative could be prepared which would be converted in the body to an active drug. Formaldehyde is a flammable, colourless gas which cannot be used directly as medicine so a stable hexamine is used. In acidic pH, hexamine is hydrolyzed to formaldehyde and ammonia which acts as a urinary antiseptic.
Ideal quality of prodrug:
A perfect prodrug must contain the following requirements. It is less effective or inactive compared to the parent compound. The pro-moiety must be cleared inside the body from prodrug. The pro-moiety should be harmless and excreted from the body[32,33].
Designing of prodrugs:
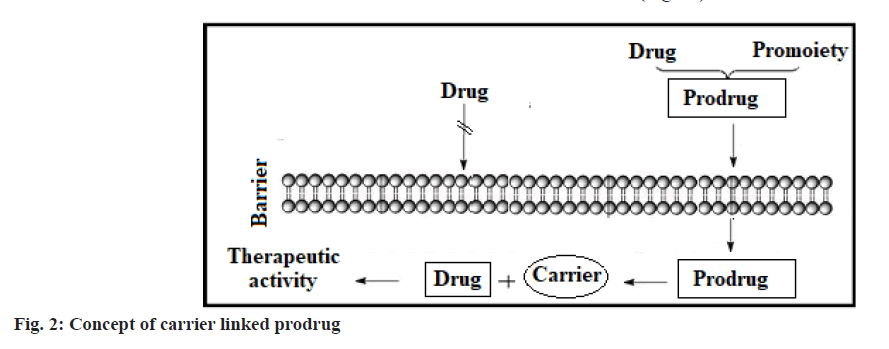
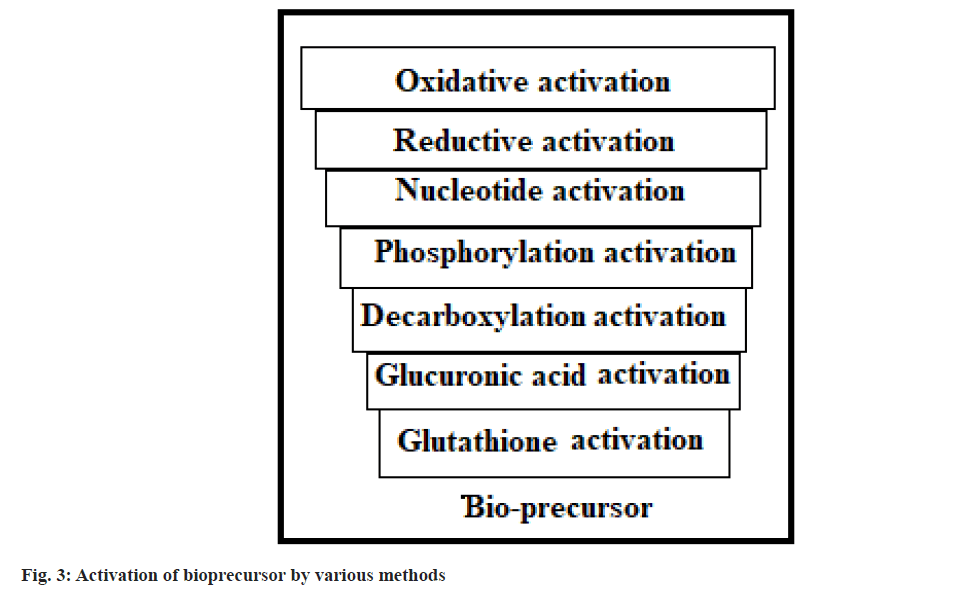
Based on the constitution, lipophilicity parameter, bio-active pathway and catalyst involved in bio-activation, the design of prodrugs is categorised into carrier-linked prodrugs and bioprecursors[34,35]. The carrier-connected prodrugs are connected to a nontoxic moiety or carrier through a covalent bond to alter or relieve their unwanted properties[36]. These prodrug molecules undergo enzymatic or chemical cleavage to discharge the active drug molecule[37] (fig. 2). Bioprecursor does not have moiety or carrier but gives an active drug molecule upon biotransformation[38]. It is changed to an active drug through oxidation, phosphorylation, reduction, nucleotide, glucuronic acid, decarboxylation and glutathione activation etc.,[39-41] (fig. 3).
Preparation:
Carboxylic acid and alcohols: The drug-containing alcohol and carboxylic acid functionalities are converted to prodrug by esterification process. Carboxylic acid possessing drug molecules can be esterified with a variety of alcohols whereas; alcohols containing drugs can be acylated with aliphatic/aromatic acid. These esters can be easily hydrolysed by esterase enzymes such as acetylcholine esterase, lipase, cholesterol esterase, carboxypeptidase, etc., present in plasma and other tissues to furnish active drugs[42].
Amines: Conversion of amines to give amide has not been commonly used as a prodrug, because of the strong stability of amide functionality and not having an amidase enzyme for metabolism in our body. A common method is to make Mannich bases as a prodrug form of amines. For example, hetacillin is a prodrug from ampicillin in which amine nitrogen and α-amino functionality have been permitted to interact with acetone to give a Mannich base[43]. Amines are also converted to azo linkage prodrug rarely.
Carbonyl moiety: The compounds containing carbonyl functionalities such as ketone and aldehydes converted to prodrugs have not found wide clinical utility. These are converted into derivatives in which the sp2 carbon of carbonyl moiety is transformed to a sp3 hybridized carbon linked to hetero atoms. These prodrugs are converted to carbonyl compounds by hydrolysis[44,45].
Bioactive Pathway
Prodrug drug designing confirmed that they are boosting the biological activities of drug molecules through increasing physicochemical properties and target selectivity. The activation of prodrugs take place by many enzymes such as carboxylesterase, butylcholinesterase, acetylcholinesterase, paraoxonase, alkaline phosphatase, matrix metalloproteinase, β-glucuronidase, plasmin, carboxypeptidase, oxidoreductases, β-lactamase and β-galactosidase etc. Here, some of the important hydrolysis processes are mentioned with suitable examples.
Carboxylesterases:
Carboxylesterases are linked to serine hydrolase enzymes and strongly hydrolyze a wide range of carbamate, esters and amide-containing drug substances. In humans, there are two types of carboxylesterases (hCE1, hCE2) are lead the drug metabolism pathway. They act as an efficient barrier to toxic materials and make easy of their removal by changing them into highly polar substances (Table 1). Here, some of the prodrugs are metabolised by carboxylesterases as mentioned below[46].
| Substrate | Name of enzyme | Hydrolysis product | Product activity |
|---|---|---|---|
| Capecitabine | hCE1 | 5'deoxy-5-flurocytidine | Inactive |
| Cocaine | hCE1 | benzoylecgonine | Inactive |
| Oseltamivir | hCE1 | oseltamivir carboxylate | Active |
| Tenofovir disoproxil | hCE2 | tenofovir | Active |
| Azilsartan medoxomil | hCE2 | azilsartan | Active |
| Propranolol ester | hCE2 | Propranolol | Active |
Table 1: Bioconversion of Various Prodrugs Bby Carboxylesterase Enzyme
Acetylcholinesterase (AChE):
AChE is an extrinsic membrane-bound enzyme that projects into the synapse. It can be able to quickly metabolised by acetylcholine at neuromuscular junctions and cholinergic synapses (Table 2). It is involved in the removal of ACh discharge from the presynaptic nerve process and is significant for protecting natural cholinergic action[47].
| Substrate | Name of enzyme | Hydrolysis product | Product activity |
|---|---|---|---|
| Butyryl acyclovir | AchE | Acyclovir | Active |
| Lovastatin | AchE | Hydroxy acid form | Active |
| Temocapril | AchE | Temocapril diacid | Active |
| CPT-11 | AchE | 7-ethyl-10hydroxycamplothecin | Active |
| Dipivefrin | AchE | Epinephrin | Active |
Table 2: Bioconversion of Various Prodrugs by Acetylcholinesterase Enzyme
Butyrylcholinesterase (BChE):
BChE is chiefly present in plasma as well as muscle, brain, kidney, retina, intestine and placenta. It is more in plasma, so often called serum cholinesterase. However, their biological role remains impossible to differentiate (Table 3). BChE can be metabolised into a huge number of prodrug-containing ester functional groups. It has a broader substrate specificity than acetylcholine esterase[48].
| Substrate | Name of enzyme | Hydrolysis product | Product activity |
|---|---|---|---|
| Bambuterol | BchE | Terbutaline | Active |
| Isosorbide Diaspirinate | BchE | Salicylic acid and isosorbide | Active |
| Methylprednisolone acetate | BchE | Methylprednisolone | Active |
| Dipivefrin Hydrochloride | BchE | Epinephrin | Active |
| Propranolol ester | BchE | Propranolol | Active |
Table 3: Bioconversion of Various Prodrugs by Butyrylcholinesterase Enzyme
Paraoxonase:
It is a member of hydrolases that comprise PON1, PON2, and PON3. PON1 is a chief esterase that can stimulate the hydrolysis of various organophosphates and arylesterase. PON2 and PON3 have 60 % chain similarity with PON1, but, they contain very some degree of arylesterase behaviour (Table 4). Human PON1 is produced in the liver and reaches into the blood. Whereas, PON3 is synthesized in the liver and has little amount in the kidney tissues[49].
| Substrate | Name of enzyme | Hydrolysis product | Product activity |
|---|---|---|---|
| Olmesartan medoxomil | hPON1 | Olmesartan and diketone compounds | Active |
| Prulifoxacin | hPON1 | Ulifoxacin | Active |
| Spironolactone | hPON1 | 7α-thiomethylspironolactone and canrenone | Active |
| lovastatin | hPON1 | beta-hydroxy acid metabolite | Active |
| mevastatin | hPON1 | Lactone ring compound | Active |
Table 4: Bioconversion of Various Prodrugs by Paraoxonase Enzyme
Matrix metalloproteinase:
They are known as matrixes, zinc-dependent endopeptidase enzymes consisting of more than 25 associates categorised based on the specificity and structure of the molecule (Table 5). They participate in a significant function in the degradation of extracellular matrix substances such as collagen, fibronectin, laminin, elastin and a variety of proteins etc. They need proteolytic enzymes to make their active forms[50].
| Substrate | Name of enzyme | EC number | Product activity |
|---|---|---|---|
| Collagens (1,2, 3, 7, 8, and 10) | MMP-1 | 3.4.24.7 | Active |
| Collagens (4, 5, 7, and 10) | MMP-2 | 3.4.24.24 | Active |
| Collagens (3, 4, 5 and 9) | MMP-3 | 3.4.24.17 | Active |
| Collagens (1, 2, 3, 5, 7, 8, and 10) | MMP-8 | 3.4.24.34 | Active |
| Collagens (4, 5, 7, 10, and 14) | MMP-9 | 3.4.24.35 | Active |
| Collagens (1, 2, 3, 4, 9, 10, and 14) | MMP-13 | - | Active |
| Collagens (1, 2 and 3) | MMP-14 | 3.4.24.80 | Active |
Table 5: Bioconversion of Various Prodrugs by Matrix Metalloproteinase Enzyme
Alkaline Phosphatase (AP):
Alkaline phosphatase is a metalloenzyme with wide substrate specificity. It encourages the taking away of a phosphate moiety from various classes of compounds such as alkaloids, nucleotides and proteins. AP is present in a lot of tissues, with the maximum expression in the hepatic cells (Table 6). The degree of difference in the number of AP isozymes in bone and liver has been utilized as a diagnostic indicator for a variety of renal dysfunction and cancer diseases[51].
| Substrate | Name of enzyme | Hydrolysis product | Product activity |
|---|---|---|---|
| Amifosline(13, WR-2721) | Alkaline phosphatase | WR-1065 | Active |
| 125IQ2-P | Alkaline phosphatase | 125IQ2-OH | Active |
| Fosfluconazole | Alkaline phosphatase | Fluconazole | Active |
| Fosphenytoin | Alkaline phosphatase | Phenytoin | Active |
| Mitomycin phosphate | Alkaline phosphatase | Mitomycin | Active |
Table 6: Bioconversion of Various Prodrugs by Alkaline Phosphate Enzyme
β-glucuronidase:
The β-glucuronidase is a lysosomal enzyme that acts a vital function in the metabolism of mucopolysaccharides, such as chondroitin sulfate, dermatan sulfate and heparan sulfate (Table 7). The action of β-glucuronidase is needed for the reforming of the extracellular matrix components and changes in the β-glucuronidase cause an atypical lysosomal storage disease described as mucopolysaccharidosis[52].
| Substrate | Name of enzyme | Hydrolysis product | Product activity |
|---|---|---|---|
| Glucuronide prodrugs of pacilitaxel | β-glucuronidase | pacilitaxel | Active |
| Glucuronide prodrugs of 5-Fluro uracil | β-glucuronidase | 5-Fluro uracil | Active |
| Glucuronide prodrugs of 9-amino-Campthotecin | β-glucuronidase | 9-amino-Campthotecin | Active |
| DOX-GA3 | β-glucuronidase | doxorubicin | Active |
| HMR1826 | β-glucuronidase | Doxorubicin | Active |
Table 7: Bioconversion of Various Prodrugs by Β-Glucuronidase Enzyme
Latest Research on Prodrugs
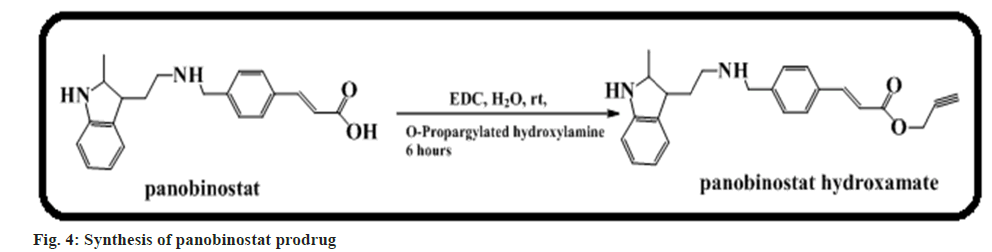
Rubio-Ruiz et al.[53] narrated that O-propargylation of the hydroxamate moiety of the strong Histone Deacetylases (HDAC) inhibitor panobinostat prodrug led to an immeasurable drop of its antitumour activity >500 fold (fig. 4). The authors also demonstrated that this prodrug is transformed into panobinostat in the existence of gold catalysts in cell culture and in vitro[53].
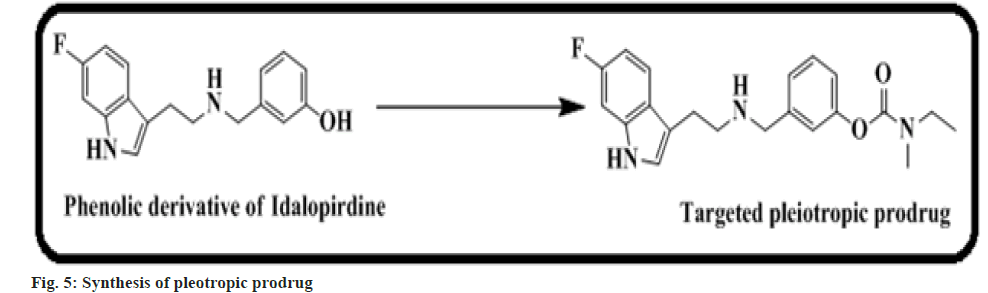
Toublet et al.[54] found that BChE might be believed as a putative target for the treatment of Alzheimer’s Disease (AD). A pleiotropic drug recognized as a multi-target ligands is presently equipped to attempt AD. In this work, we prepared a pleiotropic carbamate 7 prodrug, which acts as a covalent inhibitor of BChE (fig. 5). In silico docking and in vitro study results are parallel with the first in vivo results that show a guarantee in restoring functioning memory[54].
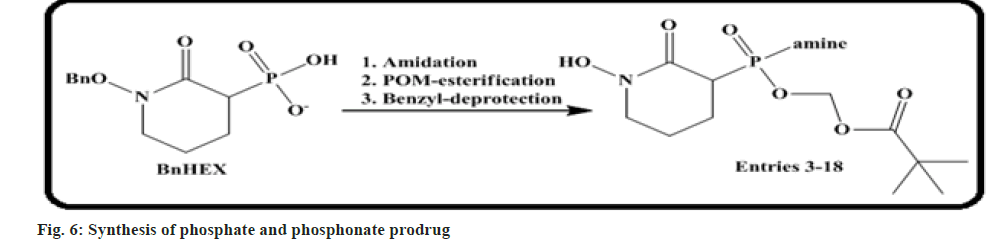
Yan et al.[55] carried out that drugs holding a single P-N bond (phosphate and phosphonates) are often employed in prodrug design to increase cell permeability of these anionic functionalities. The P-N bond is hydrolysed by phosphoramidases to liberate the effective drug molecules in vivo.
Here author narrates a new mono-amidation approach to phosphonate-containing glycolysis inhibitors and demonstrates that a diverse panel of phosphonoramidases may be quickly formed for in vitro screening. We illustrated that in contrast to the canonical benzylamine or L-alanine moieties which have earlier been reported as an efficacious prodrug, little and lengthy-chain aliphatic amines show better drug release efficacy for our phosphate inhibitors (fig. 6).
Thiabaud et al.[56] examined gadolinium (III) texaphyrinplatinum (IV) conjugates are highly effective antiproliferative properties in vitro against mouse and human cell cancer lines. Compared to the current platinum clinical standard, a lead Gd (III) texaphyrin-Pt (IV) prodrug rising from this progress effort was found to be more effective in subcutaneous route in mouse models involving both cell-derived xenografts and platinumresistant patient-derived xenografts. Based on the present study, we conclude that mellotexaphyrin- Pt conjugates may have considerable potential as anticancer agents.
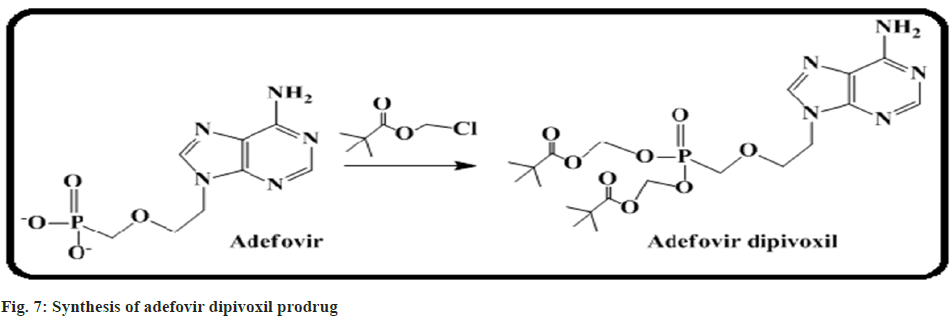
Hughes et al.[57] discussed the safety and pharmacokinetic study of the antiviral agent adefovir dipivoxil in children infected with Human Immunodeficiency Virus (HIV). It is a powerful inhibitor of retroviruses including HIV. Two dosages of the adefovir dipivoxil were assessed in a phase I study with children having HIV. Here, a total of fourteen children were divided into different groups ranging from 6 mo to 18 y. Eight patients were administered 1.5 mg of adefovir dipivoxil per kg of body weight and 6 patients received 3 mg per kg respectively. No significant adverse events were encountered (fig. 7).
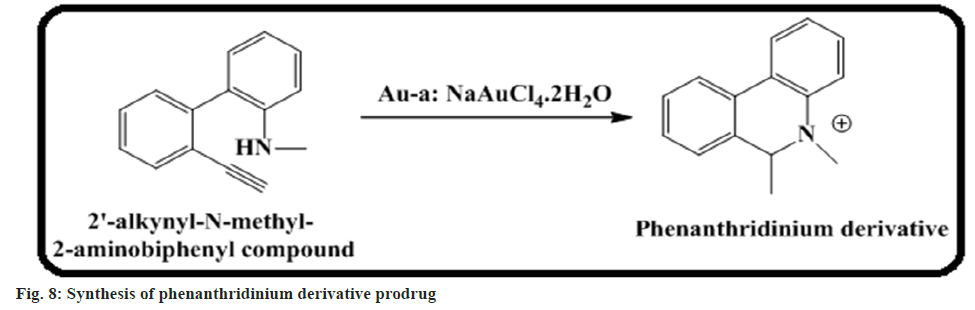
Chang et al.[58] demonstrated phenanthridinium derivatives of prodrug activation by gold. It is an upcoming technique in targeted drug delivery through metal. We introduced this approach based on the activation of a phenanthridinium-based prodrug through hydroamination under in vivo conditions. To build the prodrug compatible with the body, a gold Artificial Metalloenzyme (ArM) is made up of human serum albumin and triggers prodrug activation rather than the free gold metal complex (fig. 8).
Mohammed et al.[59] found a new prodrug Methotrexate (MTX) with chitosan and studied its bioactivity evaluation. The work intended to make an anti-colon prodrug loading into a biopolymer. Chitosan was obtained from the scales of local fish by using a standard protocol. The MTX was then loaded into chitosan to prepare chitosanmethotrexate conjugates as colon cancer prodrugs. There are 3 types of cancer cells such as MCF- 7, MDA-MB-453 and MDA-MB-231 breast cancer cell lines were committed to investigating the antitumour activity of CS-MTX by using tetrazolium assay. The results pointed out that the survival possibility of human breast tumour cell lines was reduced and proved that CS-MTX was a lesser amount toxic than the MTX mother drug.
Rana et al.[60] investigated Symbiotic Prodrugs (SymProDs) that targeting of CDK and NFκB. To test this design, we synthesised SymProDs using NFκB inhibitors and CDK inhibitors. Each drug acts as a pro-moiety of the each other and eliminates the adverse effect. The mentioned prodrug is 200-fold less active than CDK inhibitors in the in vitro method. Against ovarian cancer cell lines study revealed that energy trends of the SymProDs mirrored those of the single treatments suggesting their dissociation in cells. Our outcomes suggest that prodrugs offer a productive path for progressing the compounds and can be engaged as dual-targeting drugs.
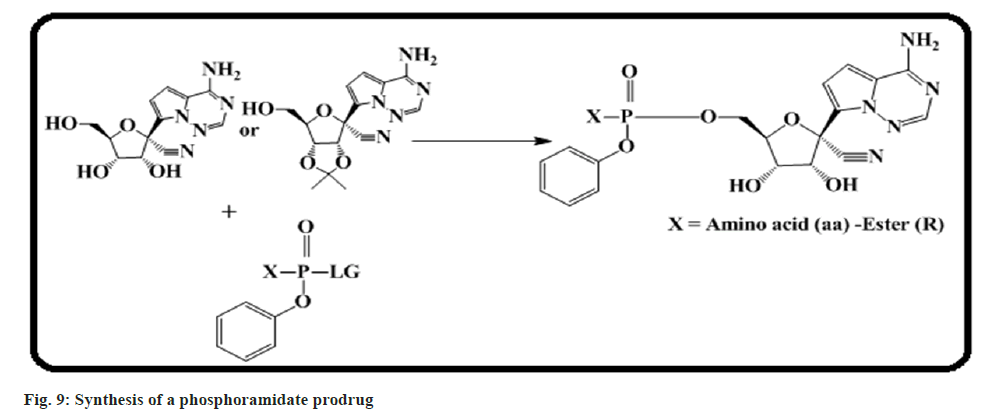
Siegel et al.[61] designed and synthesized a phosphoramidate prodrug for the treatment of emerging Ebola viral (EBOV) disease. A focused screening and optimization of lead molecules effort identified 4b (GS-5734) with antiEBOV activity (fig. 9). It has been tested in a non-human-primate EBOV challenge model. The result suggested that there is a 100 % survival of infected animals after dosing of 4b (GS-5734) with 10 mg/kg, once daily.
Conclusion
The concept of prodrug has found several useful therapeutic applications and acts as a significant alternative to solve pharmaceutical and pharmacokinetic-related problems of drug molecules. The growing proportion of prodrugs indicates the significant role of the prodrug in modern medicine. These prodrugs are activated by enzymes or/and chemical reactions. To the best of our knowledge, this review has entirely analyzed the complete information of prodrugs in all aspects such as design, synthesis, development, bioactive pathway and their recent therapeutic applications. It is the right time to consider the prodrug due to the wide variety of therapeutic applications including to treat of neglected diseases.
Acknowledgements:
All authors wish to express their gratitude to GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, India, for providing research facilities.
Conflict of interest
The authors declare no competing interests.
References
- Stella VJ. Prodrugs: My initial exploration and where it led. J Pharm Sci 2020;109(12):3514-23.
[Crossref] [Google Scholar] [PubMed]
- Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C. Prodrug approach: An overview of recent cases. Eur J Med Chem 2017;127:810-827.
[Crossref] [Google Scholar] [PubMed]
- Parise Filho R, Polli MC, Barberato Filho S, Garcia M, Ferreira EI. Prodrugs available on the Brazilian pharmaceutical market and their corresponding bioactivation pathways. Braz J Pharm Sci 2010;46:393-420.
- Mishra AP, Chandra S, Tiwari R, Srivastava A, Tiwari G. Therapeutic potential of prodrugs towards targeted drug delivery. Open Med Chem J 2018;12:111.
[Crossref] [Google Scholar] [PubMed]
- Yao Q, Lin F, Fan X, Wang Y, Liu Y, Liu Z, et al. Synergistic enzymatic and bioorthogonal reactions for selective prodrug activation in living systems. Nat Commun 2018;9(1):5032.
[Crossref] [Google Scholar] [PubMed]
- Qandil AM. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), more than meets the eye: A critical review. Int J Mol Sci 2012;13(12):17244-17274.
[Crossref] [Google Scholar] [PubMed]
- Pop E, Rachwal S, Vlasak J, Biegon A, Zharikova A, Prokai L. In vitro and in vivo study of water-soluble prodrugs of dexanabinol. J Pharm Sci 1999;88(11):1156-1160.
[Crossref] [Google Scholar] [PubMed]
- Lehouritis P, Stanton M, McCarthy FO, Jeavons M, Tangney M. Activation of multiple chemotherapeutic prodrugs by the natural enzymolome of tumour-localised probiotic bacteria. J Control Release 2016;222:9-17.
[Crossref] [Google Scholar] [PubMed]
- Mehellou Y, Rattan HS, Balzarini J. The ProTide prodrug technology: From the concept to the clinic: Miniperspective. J Med Chem 2017;61(6):2211-2226.
[Crossref] [Google Scholar] [PubMed]
- Ortiz de Montellano PR. Cytochrome P450-activated prodrugs. Future Med Chem 2013;5(2):213-228.
[Crossref] [Google Scholar] [PubMed]
- Vinarov Z, Abrahamsson B, Artursson P, Batchelor H, Berben P, Bernkop-Schnürch A, et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv Drug Deliv Rev 2021;171:289-331.
[Crossref] [Google Scholar] [PubMed]
- Mukerabigwi JF, Yin W, Zha Z, Ke W, Wang Y, Chen W, et al. Polymersome nanoreactors with tumor pH-triggered selective membrane permeability for prodrug delivery, activation, and combined oxidation-chemotherapy. J Control Release 2019;303:209-222.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Tang W. Drug metabolism in drug discovery and development. Acta Pharm Sin B 2018;8(5):721-732.
[Crossref] [Google Scholar] [PubMed]
- Rautio J, Meanwell NA, Di L, Hageman MJ. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov 2018;17(8):559-587.
[Crossref] [Google Scholar] [PubMed]
- Ren K, Dusad A, Yuan F, Yuan H, Purdue PE, Fehringer EV, et al. Macromolecular prodrug of dexamethasone prevents particle-induced peri-implant osteolysis with reduced systemic side effects. J Control Release 2014;175:1-9.
[Crossref] [Google Scholar] [PubMed]
- Karaman R. Prodrugs design based on inter andintramolecular chemical processes. Chem Biol Drug Des 2013;82(6):643-668.
[Crossref] [Google Scholar] [PubMed]
- Shah K, Gupta JK, Chauhan NS, Upmanyu N, Shrivastava SK, Mishra P. Prodrugs of NSAIDs: A review. Open Med Chem J 2017;11:146.
[Crossref] [Google Scholar] [PubMed]
- Qandil AM. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), more than meets the eye: A critical review. Int J Mol Sci 2012;13(12):17244-17274.
[Crossref] [Google Scholar] [PubMed]
- Sandros MG, Sarraf CB, Tabrizian M. Prodrugs in cardiovascular therapy. Molecules 2008;13(5):1156-1178.
[Crossref] [Google Scholar] [PubMed]
- De Clercq E, Field HJ. Antiviral prodrugs–the development of successful prodrug strategies for antiviral chemotherapy. Br J Pharmacol 2006;147(1):1-1.
[Crossref] [Google Scholar] [PubMed]
- Jubeh B, Breijyeh Z, Karaman R. Antibacterial prodrugs to overcome bacterial resistance. Molecules 2020;25(7):1543.
[Crossref] [Google Scholar] [PubMed]
- Najjar A, Karaman R. The prodrug approach in the era of drug design. Expert Opin Drug Deliv 2019;16(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Ohwada J, Tsukazaki M, Hayase T, Oikawa N, Isshiki Y, Fukuda H, et al. Design, synthesis and antifungal activity of a novel water soluble prodrug of antifungal triazole. Bioorg Med Chem Lett 2003;13(2):191-196.
- Maslah H, Skarbek C, Pethe S, Labruère R. Anticancer boron-containing prodrugs responsive to oxidative stress from the tumor microenvironment. Eur J Med Chem 2020;207:112670.
[Crossref] [Google Scholar] [PubMed]
- Peesa JP, Yalavarthi PR, Rasheed A, Mandava VB. A perspective review on role of novel NSAID prodrugs in the management of acute inflammation. J Acute Dis 2016;5(5):364-381.
- Nicolas J. Drug-initiated synthesis of polymer prodrugs: Combining simplicity and efficacy in drug delivery. Chem Mater 2016;28(6):1591-606.
[Crossref] [Google Scholar] [PubMed]
- Sansom GN, Kirk NS, Guise CP, Anderson RF, Smaill JB, Patterson AV, et al. Prototyping kinase inhibitor-cytotoxin anticancer mutual prodrugs activated by tumour hypoxia: A chemical proof of concept study. Bioorg Med Chem Lett 2019;29(10):1215-1219.
[Crossref] [Google Scholar] [PubMed]
- Lesniewska-Kowiel MA, Muszalska I. Strategies in the designing of prodrugs, taking into account the antiviral and anticancer compounds. Eur J Med Chem 2017;129:53-71.
[Crossref] [Google Scholar] [PubMed]
- Bildstein L, Pili B, Marsaud V, Wack S, Meneau F, Lepêtre-Mouelhi S, et al. Interaction of an amphiphilic squalenoyl prodrug of gemcitabine with cellular membranes. Eur J Pharm Biopharm 2011;79(3):612-620.
[Crossref] [Google Scholar] [PubMed]
- Powell MF, Magill A, Chu N, Hama K, Mau CI, Foster L, et al. Chemical and enzymatic degradation of ganciclovir prodrugs: Enhanced stability of the diadamantoate prodrug under acid conditions. Pharm Res 1991;8:1418-23.
[Crossref] [Google Scholar] [PubMed]
- Barot M, Bagui M, R Gokulgandhi M, K Mitra A. Prodrug strategies in ocular drug delivery. Med Chem 2012;8(4):753-768.
[Crossref] [Google Scholar] [PubMed]
- Markovic M, Ben-Shabat S, Dahan A. Prodrugs for improved drug delivery: Lessons learned from recently developed and marketed products. Pharmaceutics 2020;12(11):1031.
[Crossref] [Google Scholar] [PubMed]
- Hajnal K, Gabriel H, Aura R, Erzsébet V, Blanka SS. Prodrug strategy in drug development. Acta Marisiensis Seria Med 2016;62(3):356-362.
- Testa B. Prodrugs: Bridging pharmacodynamic/pharmacokinetic gaps. Curr Opin Chem Biol 2009;13(3):338-344.
[Crossref] [Google Scholar] [PubMed]
- Jornada DH, dos Santos Fernandes GF, Chiba DE, de Melo TR, Dos Santos JL, Chung MC. The prodrug approach: A successful tool for improving drug solubility. Molecules 2015;21(1):42.
[Crossref] [Google Scholar] [PubMed]
- Kumari N, Sapra B. Ocular prodrugs: Attributes and challenges. Asian J Pharm Sci 2021;16(2):175-191.
[Crossref] [Google Scholar] [PubMed]
- Wiemer AJ. Metabolic efficacy of phosphate prodrugs and the remdesivir paradigm. ACS Pharmacol Transl Sci 2020;3(4):613-626.
[Crossref] [Google Scholar] [PubMed]
- Fattahi N, Shahbazi MA, Maleki A, Hamidi M, Ramazani A, Santos HA. Emerging insights on drug delivery by fatty acid mediated synthesis of lipophilic prodrugs as novel nanomedicines. J Control Release 2020;326:556-598.
[Crossref] [Google Scholar] [PubMed]
- Pertusat F, Serpi M, McGuigan C. Medicinal chemistry of nucleoside phosphonate prodrugs for antiviral therapy. Antivir Chem Chemother 2012;22(5):181-203.
[Crossref] [Google Scholar] [PubMed]
- Chen KC, Cheng TL, Leu YL, Prijovich ZM, Chuang CH, Chen BM, et al. Membrane-localized activation of glucuronide prodrugs by β-glucuronidase enzymes. Cancer Gene Ther 2007;14(2):187-200.
[Crossref] [Google Scholar] [PubMed]
- Ruzza P, Calderan A. Glutathione Transferase (GST)-activated prodrugs. Pharmaceutics 2013;5(2):220-231.
[Crossref] [Google Scholar] [PubMed]
- Mizrahi B, Domb AJ. Anhydride prodrug of ibuprofen and acrylic polymers. AAPS PharmSciTech 2009;10:453-458.
[Crossref] [Google Scholar] [PubMed]
- Piplani M, Rajak H, Sharma PC. Synthesis and characterization of N-Mannich based prodrugs of ciprofloxacin and norfloxacin: In vitro anthelmintic and cytotoxic evaluation. J Adv Res 2017;8(4):463-470.
[Crossref] [Google Scholar] [PubMed]
- Kumari R, Sunil D, Ningthoujam RS, Kumar NA. Azodyes as markers for tumor hypoxia imaging and therapy: An up-to-date review. Chem Biol Interact 2019;307:91-104.
[Crossref] [Google Scholar] [PubMed]
- Devarajan-Ketha H, Sloan KB. N, N′-Dialkylaminoalkylcarbonyl (DAAC) prodrugs and Aminoalkylcarbonyl (AAC) prodrugs of 4-hydroxyacetanilide and naltrexone with improved skin permeation properties. Bioorg Med Chem Lett 2011;21(13):4078-4082.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Zou L, Jin Q, Hou J, Ge G, Yang L. Human carboxylesterases: A comprehensive review. Acta Pharm Sin B 2018;8(5):699-712.
[Crossref] [Google Scholar] [PubMed]
- Toublet FX, Lecoutey C, Lalut J, Hatat B, Davis A, Since M, et al. Inhibiting acetylcholinesterase to activate pleiotropic prodrugs with therapeutic interest in Alzheimer’s disease. Molecules 2019;24(15):2786.
[Crossref] [Google Scholar] [PubMed]
- Mata G, do Rosário VE, Iley J, Constantino L, Moreira R. A carbamate-based approach to primaquine prodrugs: Antimalarial activity, chemical stability and enzymatic activation. Bioorg Med Chem 2012;20(2):886-892.
[Crossref] [Google Scholar] [PubMed]
- Ishizuka T, Fujimori I, Kato M, Noji-Sakikawa C, Saito M, Yoshigae Y, et al. Human carboxymethylenebutenolidase as a bioactivating hydrolase of olmesartan medoxomil in liver and intestine. J Biol Chem 2010;285(16):11892-02.
[Crossref] [Google Scholar] [PubMed]
- Samuelson LE, Scherer RL, VanSaun MN, Fan KH, Dozier EA, Carter KJ, et al. New tools for the quantitative assessment of prodrug delivery and neurotoxicity. Neurotoxicology 2015;47:88-98.
[Crossref] [Google Scholar] [PubMed]
- Xie A, Hanif S, Ouyang J, Tang Z, Kong N, Kim NY, et al. Stimuli-responsive prodrug-based cancer nanomedicine. EBioMedicine 2020;56.
[Crossref] [Google Scholar] [PubMed]
- Tranoy-Opalinski I, Legigan T, Barat R, Clarhaut J, Thomas M, Renoux B, et al. β-Glucuronidase-responsive prodrugs for selective cancer chemotherapy: An update. Eur J Med Chem 2014;74:302-13.
[Crossref] [Google Scholar] [PubMed]
- Rubio-Ruiz B, Perez-Lopez AM, Sebastián V, Unciti-Broceta A. A minimally-masked inactive prodrug of panobinostat that is bioorthogonally activated by gold chemistry. Bioorg Med Chem 2021;41:116217.
[Crossref] [Google Scholar] [PubMed]
- Toublet FX, Lalut J, Hatat B, Lecoutey C, Davis A, Since M, et al. Pleiotropic prodrugs: Design of a dual butyrylcholinesterase inhibitor and 5-HT6 receptor antagonist with therapeutic interest in Alzheimer’s disease. Eur J Med Chem 2021;210:113059.
[Crossref] [Google Scholar] [PubMed]
- Yan VC, Pham CD, Arthur K, Yang KL, Muller FL. Aliphatic amines are viable pro-drug moieties in phosphonoamidate drugs. Bioorg Med Chem Lett 2020;30(24):127656.
[Crossref] [Google Scholar] [PubMed]
- Thiabaud G, He G, Sen S, Shelton KA, Baze WB, Segura L, et al. Oxaliplatin Pt (IV) prodrugs conjugated to gadolinium-texaphyrin as potential antitumor agents. Proc Natl Acad Sci U S A. 2020;117(13):7021-9.
[Crossref] [Google Scholar] [PubMed]
- Hughes WT, Shenep JL, Rodman JH, Fridland A, Willoughby R, Blanchard S, et al. Single-dose pharmacokinetics and safety of the oral antiviral compound adefovir dipivoxil in children infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother 2000;44(4):1041-6.
[Crossref] [Google Scholar] [PubMed]
- Chang TC, Vong K, Yamamoto T, Tanaka K. Prodrug activation by gold artificial metalloenzyme-catalyzed synthesis of phenanthridinium derivatives via hydroamination. Angew Chem Int Ed Engl 2021;133(22):12554-62.
[Crossref] [Google Scholar] [PubMed]
- Mohammed MO, Alkubaisi HM, Haj NQ. A new prodrug and bioactivity evaluation of methotrexate based on Chitosan. Heliyon 2020;6(6):e04223.
[Crossref] [Google Scholar] [PubMed]
- Rana S, Kour S, Sonawane YA, Robb CM, Contreras JI, Kizhake S, Zahid M, Karpf AR, Natarajan A. Symbiotic prodrugs (SymProDs) dual targeting of NFkappaB and CDK. Chem Biol Drug Des 2020;96(2):773-84.
[Crossref] [Google Scholar] [PubMed]
- Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo [2, 1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J Med Chem 2017;60(5):1648-