- *Corresponding Author:
- Shanshan Fan
Department of Ophthalmology, The Affiliated Hospital of Weifang Medical University, Weifang, Shandong 261031, China
E-mail: 13863648701@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “212-218” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the clinical effect of travoprost and timolol eye drops for senile glaucoma. This study selected 88 individuals having senile glaucoma admitted to the Affiliated Hospital of Weifang Medical University hospital for treatment between January 2020 and January 2022. Depending on treatment regimens, they were assigned to an observation group who were given travoprost (n=47) and control group who were given timolol eye drops (n=41). The therapeutic effects, visual field defects, 24 h peak intraocular pressure, binocular optic disc parameters, adverse effects and quality of life of the two groups were analyzed. Patients in the observation group showed notably better treatment outcomes when compared with the control patients. Obvious post-treatment improvements of visual field defects, 24 h peak intraocular pressure, binocular optic disc parameters and quality of life were observed in both groups, especially in the observation group; no significant inter-group difference was determined in adverse effects. Travoprost is superior to timolol eye drops to treat primary glaucoma in older adults, which can effectively ameliorate intraocular pressure and visual field defects, with less adverse effects and high safety. Its clinical application can be beneficial and is therefore recommended to be further popularized clinically.
Keywords
Aortic dissection, bioinformatics, biomarkers, differentially expressed gene, protein-protein interaction
As a medical condition that mainly causes optic nerve damage, glaucoma majorly affects the middle-aged and elderly people and is insidious in symptoms and slow in progression. As a result, its optimal treatment period is missed without realizing the disease in most cases, causing optic neuropathy and even inducing blindness[1,2]; selecting appropriate treatments is therefore crucial. Glaucoma is characterized by pathologic high Intraocular Pressure (IOP) or poor blood perfusion of the Optic Disc (OD) complicated with visual dysfunction, which can be classified as either congenital, secondary or primary, among which primary glaucoma is further subdivided into angle-closure and Open-Angle Glaucoma (OAG), with the glaucoma damage degree representing the degree of optic nerve damage in glaucoma[3,4].
IOP control is the key to glaucoma treatment, which can be carried out through drug therapy, laser therapy and surgical treatment[5]. Surgery or laser treatment is the preferred choice for many glaucoma patients as the related technology advances. But drug therapy, being a commonly used initial and supplementary therapy following surgical or laser treatment, still occupies a crucial position in glaucoma treatment[6], with topical eye drops being the major therapy. Timolol (TIM), a Beta (β)-adrenoreceptor antagonist, reduces IOP in primary OAG and exerts antihypertensive effects on secondary glaucoma, ocular hypertension, partial angle-closure glaucoma and glaucoma that does not respond to drugs or surgery, with its mechanism of action in lowering IOP reported to be mainly due to the reduction of aqueous humor production[7,8]. Travoprost (TRA) is a novel medication with relatively few head-to-head studies for comparing drug efficacy and safety. Additionally, there is limited analysis regarding their use among elderly glaucoma patients. Therefore, selection of these two drugs mainly aimed at providing more data support for clinical treatment options for elderly glaucoma patients. On the other hand TRA is a selective Prostaglandin F (FP) receptor agonist that can reduce IOP by promoting aqueous humor outflow through the uveoscleral pathway[9]. Yet, little is known about the difference of TRA vs. TIM eye drops for the treatment of senile glaucoma.
Based on this, this study attempts to comparatively analyze the effectiveness and safety of TRA vs. TIM eye drops in senile glaucoma, so as to render more clinical suggestions for the treatment of senile glaucoma from the perspective of drug therapy.
Materials and Methods
General information:
This is a retrospective analysis that included 88 elderly glaucoma patients from The Affiliated Hospital of Weifang Medical University hospital who were admitted between January 2020 and January 2022, including 45 male patients and 43 female patients aged (68.11±2.32) y on an average. They were grouped into observation group (TRA intervention) with 47 individuals and control group (TIM intervention) with 41 individuals depending on their treatment schemes. The ethics committee approved the study (Approval no: 2020-01) which followed the recommendations of Declaration of Helsinki.
Inclusion criteria:
Patients who were diagnosed with OAG, having IOP >21 mmHg, glaucomatous OD due to insufficient blood supply, angle opening, and Visual Field Defects (VFDs) or retinal nerve fiber layer defects; patients having no abnormalities of the cornea on slit-lamp examination; patients having >0.3 best corrected visual acuity and patients who did not use medication such as carbonic anhydrase inhibitors, β-blockers or cholinergic inhibitors prior (i.e., 2 w before treatment) were included in this study.
Exclusion criteria:
Patients who had undergone ophthalmic surgery previously; patients with improper medicationinduced lesions; patients who had the history of acute angle-closure glaucoma or angle closure previously; patients having conjunctivitis, keratitis, uveitis and other acute and chronic eye diseases; pregnant and lactating patients and patients having a history of allergy to the medication used in the study were excluded in this study.
Treatment method:
Control group patients were given 1 drop of TIM eye drops (Hubei Qianjiang Pharmaceutical Co., Ltd., having Saudi Food and Drug Authority (SFDA) approval no. H20065130) twice a day to the affected eye (i.e., at 8 AM and 6 PM). The lacrimal sac area was compressed for 5 min following the application of the eye drops; the course of treatment was 4 w. Similarly, the observation group was treated with TRA eye drops (0.004 %) (Alcon (China) Ophthalmic Product Co., Ltd.), which were given at 8 PM every day, with a dose of 1 drop each time; after dropping the drug into the lower conjunctival sac, the lacrimal sac area was compressed for >3 min.
Endpoints:
Efficacy: The effectiveness of the therapeutic effects was assessed using the criteria for comparative analysis namely, marked effectiveness, effectiveness and ineffectiveness. Marked effectiveness was defined as the disappearance of basic clinical symptoms, basically recovered retinal circulation, visual acuity improvement by ≥2 lines and an expansion of visual field of 5 adjacent visual points or ≥5°. Effectiveness depicted improved clinical symptoms and retinal circulation, visual acuity improvement by 1 line, and an expansion of the visual field with <5°. Similarly, no relief or aggravation of clinical symptoms, no improvement in retinal circulation and no enhancement or even deterioration of visual acuity was considered as ineffectiveness.
Total effective rate=(marked effectiveness cases+effectiveness cases)/total cases×100 %
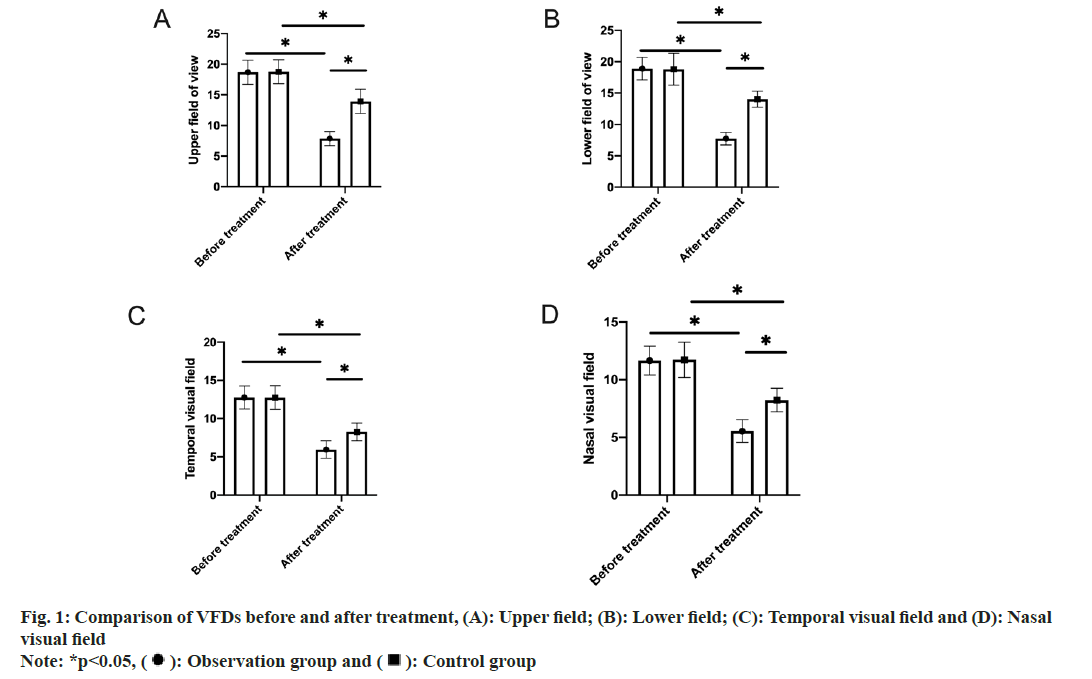
VFDs: Pre- and post-treatment VFDs, including the upper, lower, temporal and nasal visual fields, were measured.
IOP: IOP of the patients before and after 4 w of treatment was measured using a Keeler noncontact tonometer (Fuan Shanghai Enterprise Development Co., Ltd.). In the sitting position, the patient was measured for 3 consecutive times to obtain the Mean Deviation (MD).
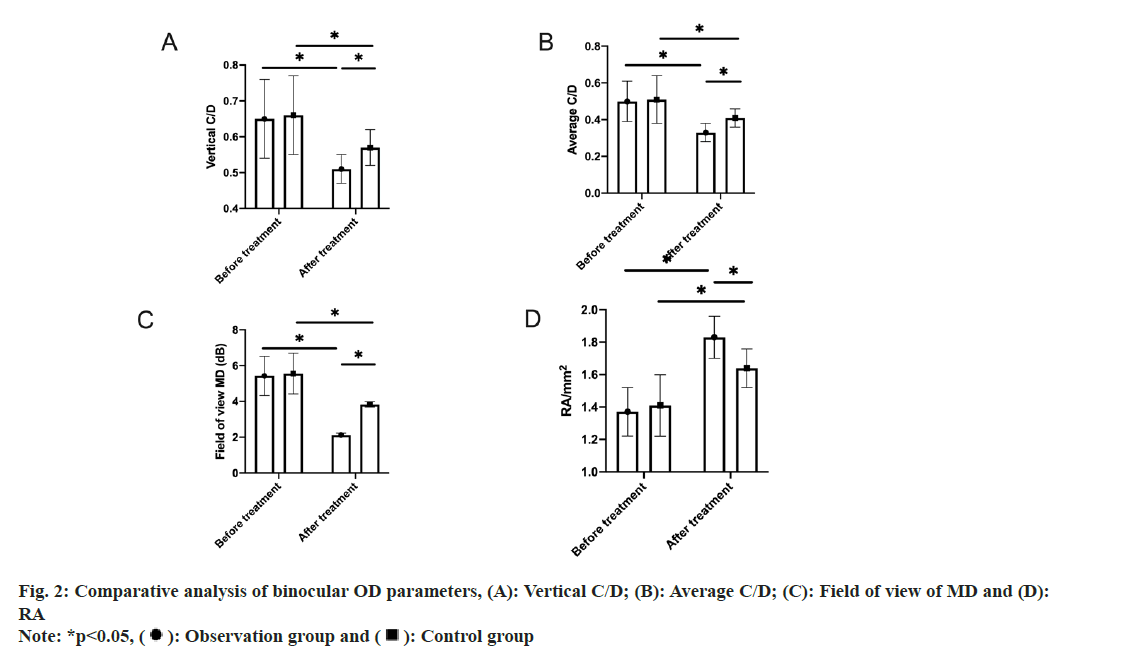
OD parameters detection: During Optical Coherence Tomography (OCT), we selected the OD scanning mode for scanning and obtained detection data in the mydriasis state. This was followed by data processing and analysis using the program that came with the device, which automatically generated binocular OD parameters like vertical Cup-to-Disc ratio (C/D), average C/D and Rim Area (RA).
Adverse Effects (AEs): AEs such as arrhythmia, cardiac acceleration, tachypnea, conjunctival congestion, etc., observed during the treatment were observed and compared between both the groups.
Quality of Life (QOL): Patients' vision-related QOL was assessed using the Chinese version of the 25-item National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25)[10]. The questionnaire included 26 items from 12 dimensions, each scored on a 6-level scale (A, B, C, D, E and F). The total score is 0-100, where higher scores indicate less damage indicating better QOL.
Statistical analysis:
Data analysis and visualization was made by Statistical Package of Social Sciences (SPSS) version 18.0 (Beijing ND Times Technology Co., Ltd.) and GraphPad Prism 6, respectively with a statistical significance indicated by p<0.05. Chisquare (χ2) tests were used for counting data; independent t-tests (inter-group) and paired t-tests before and after treatment were used for analyzing the data.
Results and Discussion
Comparison of baseline data such as gender, age, smoking history, etc., between both the groups were evaluated, which suggested comparability with a statistical significance of p>0.05 (Table 1).
| Factors | Observation group (n=47) | Control group (n=41) | t/χ2 | p |
|---|---|---|---|---|
| Gender | 0.059 | 0.808 | ||
| Male | 24 (51.06) | 22 (53.66) | ||
| Female | 23 (48.94) | 19 (46.34) | ||
| Age (y) | 0.016 | 0.899 | ||
| ≤68 | 20 (42.55) | 18 (43.90) | ||
| >68 | 27 (57.45) | 23 (56.10) | ||
| Body Mass Index (BMI) (kg/m2) | 0.002 | 0.965 | ||
| ≤ 23 | 22 (46.81) | 19 (46.34) | ||
| >23 | 25 (53.19) | 22 (53.66) | ||
| Average course of disease (y) | 4.81±1.02 | 4.89±1.00 | 0.370 | 0.712 |
| Number of affected eyes | 0.059 | 0.808 | ||
| Monocular | 23 (48.94) | 19 (46.34) | ||
| Binocular | 24 (51.06) | 22 (53.66) | ||
| Liver function indices | ||||
| Serum total protein (g/l) | 67.05±1.52 | 67.48±1.47 | 1.344 | 0.182 |
| Total bilirubin (μmol/l) | 11.34±1.34 | 10.86±1.07 | 1.838 | 0.075 |
Table 1: General data, N (%)
Comparative analysis of effectiveness was carried out, where we found that the number of patients with marked effectiveness, effectiveness and ineffectiveness were 30, 14 and 3 in the observation group, and 18, 12 and 11 in the control group, respectively. The total effective rate which was calculated was found to be 93.61 % in the observation group, higher than the 73.17 % in the control group (p<0.05) (Table 2).
| Curative effect | Observation group (n=47) | Control group (n=41) | χ2 | p |
|---|---|---|---|---|
| Marked effectiveness | 30 (63.83) | 18 (43.90) | - | - |
| Effectiveness | 14 (29.79) | 12 (29.27) | - | - |
| Ineffectiveness | 3 (6.38) | 11 (26.83) | - | - |
| Total effective rate | 44 (93.61) | 30 (73.17) | 4.606 | 0.032 |
Table 2: Comparison of curative effects, N (%)
Pre- and post-treatment IOP levels between the two groups were investigated. IOP of both the groups decreased significantly after treatment, showing a statistical difference compared with the baseline level (p<0.05). Besides, an evident difference was also identified in post-treatment IOP between groups, with an even better IOP in the observation group (p<0.05) (Table 3).
| 24 h peak IOP | Observation group (n=47) | Control group (n=41) | t | p |
|---|---|---|---|---|
| Before treatment | 25.01±0.84* | 25.2±1.29* | 0.829 | 0.410 |
| After treatment | 17.02±1.13# | 19.16±0.99# | 9.384 | <0.001 |
Note:*p<0.05 vs. #p<0.05
Table 3: Comparative analysis of 24 H Peak IOP
Subsequently, pre- and post-treatment VFDs were also studied where no significant intergroup difference was identified in pre-treatment VFDs (p>0.05). Significantly reduced ranges of the defects in all directions were found in both groups after drug therapy, with smaller VFDs in the observation group (p<0.05) (fig. 1).
Comparative analysis of binocular OD parameters between the two groups was assessed.
The two groups differed slightly in pre-treatment vertical C/D, average C/D, field of view MD and RA (p>0.05). Statistical intra-group significance was determined in all these indices in both the groups, with reduced vertical C/D, average C/D and field of view MD and elevated RA after treatment (p<0.05). Moreover, the inter-group comparison revealed the presence of significant differences after treatment, with even better OD parameters and visual field MD in the observation group (p<0.05) (fig. 2).
AEs were also examined comparatively. The number of patients with arrhythmia, cardiac acceleration, tachypnea and conjunctival congestion in the observation group were 2, 1, 0, and 2, respectively, whose incidence of AEs was found to be 10.64 %. In the control group, arrhythmia, cardiac acceleration, tachypnea and conjunctival congestion were found in 1, 1, 1, and 2 patients, respectively, with an AE rate of 12.20 %. No significant inter-group difference was determined in the AE rate (p>0.05) (Table 4).
| Adverse effect | Observation group (n=47) | Control group (n=41) | χ2 | p |
|---|---|---|---|---|
| Arrhythmia | 2 (4.25) | 1 (2.44) | - | - |
| Cardiac acceleration | 1 (2.13) | 1 (2.44) | - | - |
| Tachypnea | 0 | 1 (2.44) | - | - |
| Conjunctival hyperemia | 2 (4.25) | 2 (4.88) | - | - |
| Incidence rate | 5 (10.64) | 5 (12.20) | 0.053 | 0.818 |
Table 4: Comparative analysis of adverse effects, N (%)
Subsequently, comparative analysis of visionrelated quality of life between the two groups was studied. Compared with the baseline, the total National Eye Institute Visual Function Questionnaire (NEI VFQ)-25 score in both groups increased markedly after treatment, with a statistical intra-group difference (p<0.05). Moreover, the observation group had a notably better NEI VFQ-25 score than the control group after treatment (p<0.05) (Table 5).
| Vision-related QOL | Observation group (n=47) | Control group (n=41) | t | p |
|---|---|---|---|---|
| Before treatment | 65.11±5.04 | 66.25±4.01 | 1.162 | 0.248 |
| After treatment | 81.19±5.09 | 74.95±3.14 | 6.799 | <0.001 |
Table 5: Comparative analysis of Vision-related QOL scores
Glaucoma ranks 1st in the incidence among irreversible blinding eye diseases and IOP is currently recognized as a major risk factor for its occurrence and development. How to effectively control IOP and protect the optic nerve has become the ultimate goal of treatment[11]. At present, drugs, surgery, laser therapy and other methods are often used to treat glaucoma. Although surgical and laser treatment can play a certain therapeutic effect, they are traumatic and detrimental to patient long-term outcomes. As the disease is prone to occur among the elderly population with a huge impact on their QOL, finding effective means to prevent and treat glaucoma has become a social and clinical concern and a research hotspot[12,13].
We primarily compared the clinical effects of TRA vs. TIM eye drops for senile glaucoma and observed significantly better efficacy of TRA. TIM eye drops are commonly used clinically to treat glaucoma. As a non-selective β-receptor blocker, TIM has a certain effect in reducing IOP, but it will have a "long-term drift" effect over time, that is, the effect will gradually weaken with the extension of medication time[14]. TRA eye drops are a new prostaglandin drug for lowering IOP. As a new anti-glaucoma agent, it has been gradually applied to the clinical treatment of glaucoma in recent years and well received by patients and clinicians due to effective symptom relief and few AEs[15]. This study found that the improvement of IOP and visual field score was more obvious in the observation group, with a total effective rate up to 93.3 %, suggesting its promising application value.
We also tested a series of more comprehensive indicators for glaucoma. IOP is important in diagnosing glaucoma[16]. NEI VFQ-25 can comprehensively evaluate the quality of life related to visual function in patients with eye diseases, with well documented validity and reliability, which is suitable for condition assessment of common eye disorders[17]. OAG is pathologically characterized by OD damage and VFDs. Patients show significantly increased vertical and average C/D and other optic cup morphological parameters, while RA is obviously reduced, resulting in glaucomatous visual field damage[18].
In this study, the IOP, vertical and average C/D, and field of view MD were significantly reduced in the observation group vs. the other at each posttreatment period, with notably increased total NEI VFQ-25 score and RA, demonstrating the effectiveness of TRA in treating senile primary glaucoma. TRA has been indicated to play a role in protecting patients' vision. Glaucoma is mostly attributed to optic neurovascular regulation dysfunction, making it difficult for blood vessels to modulate the elevated blood flow when the IOP increases. TRA has a lasting control of IOP, effectively controlling IOP for >24 h and stabilizing IOP fluctuations day and night, with no such "shift” phenomenon like TIM after long-term medication[19,20]. Mechanically speaking, TRA is an isopropyl prodrug that can be quickly hydrolyzed into a bioactive free acid after eye drops, while TRA free acid is a selective prostaglandin FP receptor agonist, which, after binding to receptors, can reduce IOP by increasing aqueous outflow through the uveoscleral pathway. TRA eye drops is effective in controlling IOP for up to 24 h, especially during night[21,22], which explains our conclusions. At the same time, the AEs of both groups were mainly mild eye congestion and arrhythmia, with no serious adverse events, indicating a good safety profile of both therapies.
Conclusively, TRA has high clinical efficacy in senile primary glaucoma. Further, it can validly reduce IOP and VFDs while contributing to fewer AEs by maintaining high drug safety. Additionally, it demonstrates the clinical benefits and also suggests that it can be further promoted in clinical use. However, this study still has certain limitations. On one hand, given the small sample size, the findings require further verification through subsequent multicenter, large-sample studies. On the other hand, there are many drugs available for the clinical treatment of glaucoma, but this study only selected relatively common and innovative drugs. In future, more drugs need to be included in the research for analysis to provide more data support for glaucoma treatment.
Conflict of interests:
The authors declare no conflict of interests.
References
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA 2014;311(18):1901-11.
- He S, Stankowska DL, Ellis DZ, Krishnamoorthy RR, Yorio T. Targets of neuroprotection in glaucoma. J Ocul Pharmacol Ther 2018;34(1-2):85-106.
- Selvan H, Gupta S, Wiggs JL, Gupta V. Juvenile-onset open-angle glaucoma-A clinical and genetic update. Surv Ophthalmol 2022;67(4):1099-117.
- Sun MT, Tran M, Singh K, Chang R, Wang H, Sun Y. Glaucoma and myopia: Diagnostic challenges. Biomolecules 2023;13(3):1-11.
- Go MS, Freedman SF. Minimally invasive glaucoma surgery in childhood glaucoma. Curr Opin Ophthalmol 2022;33(2):91-6.
- Greslechner R, Helbig H. Secondary glaucoma in the context of retinal disease. Klin Monbl Augenheilkd 2022;239(9):1111-8.
[Crossref] [Google Scholar] [PubMed]
- Philippin H, Matayan E, Knoll KM, Macha E, Mbishi S, Makupa A, et al. Selective laser trabeculoplasty vs. 0.5 % timolol eye drops for the treatment of glaucoma in Tanzania: A randomised controlled trial. Lancet Glob Health 2021;9(11):1589-99.
- Chiou GC. Development of D-timolol for the treatment of glaucoma and ocular hypertension. J Ocul Pharmacol 1990;6(1):67-74.
- Denis P. Travoprost/timolol fixed combination in the management of open-angle glaucoma: A clinical review. Expert Opin Pharmacother 2011;12(3):463-71.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol 2001;119(7):1050-8.
- Schuster AK, Wagner FM, Pfeiffer N, Hoffmann EM. Risk factors for open-angle glaucoma and recommendations for glaucoma screening. Ophthalmologe 2021;118:145-52.
- Erb C, Predel HG. Relevance of arterial hypertension in primary open-angle glaucoma. Klin Monbl Augenheilkd 2014;231(2):136-43.
- Rivera JL, Bell NP, Feldman RM. Risk factors for primary open angle glaucoma progression: What we know and what we need to know. Curr Opin Ophthalmol 2008;19(2):102-6.
- Heel RC, Brogden RN, Speight TM, Avery GS. Timolol: A review of its therapeutic efficacy in the topical treatment of glaucoma. Drugs 1979;17(1):38-55.
- Gandolfi S, Paredes T, Goldberg I, Coote M, Wells A, Volksone L, et al. Comparison of a travoprost BAK-free formulation preserved with polyquaternium-1 with BAK-preserved travoprost in ocular hypertension or open-angle glaucoma. Eur J Ophthalmol 2012;22(1):34-44.
- Lin Z, Huang S, Huang P, Li C, Chen Z, Zhong Y. Concordance of 24 h intraocular pressure curve in patients with untreated unilateral primary open-angle glaucoma. Exp Ther Med 2018;16(2):1461-9.]
- Karadeniz Ugurlu S, Kocakaya Altundal AE, Altin Ekin M. Comparison of vision-related quality of life in primary open-angle glaucoma and dry-type age-related macular degeneration. Eye 2017;31(3):395-405.
- Siesky B, Wentz SM, Januleviciene I, Kim DH, Burgett KM, Rowe LW, et al. Baseline structural characteristics of the optic nerve head and retinal nerve fiber layer are associated with progressive visual field loss in patients with open-angle glaucoma. PLoS One 2020;15(8):1-11.
- Zhang XL, Qin L. Efficacy of travoprost for the treatment of patients with glaucoma. Medicine 2019;98(29):1-3.
- Eisenberg DL, Toris CB, Camras CB. Bimatoprost and travoprost: A review of recent studies of two new glaucoma drugs. Surv Ophthalmol 2002;47:105-15.
- Holmstrom S, Buchholz P, Walt J, Wickstrøm J, Aagren M. Analytic review of bimatoprost, latanoprost and travoprost in primary open angle glaucoma. Curr Med Res Opin 2005;21(11):1875-83.
- Kim JM, Sung KR, Kim HK, Park SW, Lee EJ, Jeoung JW, et al. Long-term effectiveness and safety of tafluprost, travoprost, and latanoprost in Korean patients with primary open-angle glaucoma or normal-tension glaucoma: A multicenter retrospective cohort study (LOTUS study). J Clin Med 2021;10(12):1-12.
 ): Observation group and (
): Observation group and ( ): Control group
): Control group