- *Corresponding Author:
- H. S. Maji
Department of Pharmaceutical Technology, JIS University, Kolkata-700 109, India
E-mail: hsmaji77@gmail.com
| Date of Submission | 31 October 2016 |
| Date of Revision | 14 February 2017 |
| Date of Acceptance | 22 July 2017 |
| Indian J Pharm Sci 2017;79(5):751-757 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Antihistamines belong to H1 receptor antagonist class of drugs. Pharmacologically they are classified into two categories namely first generation, mostly sedating in nature and second generation, which are less sedating and quite selective in activity. Widespread uses of antihistamines were found during microbial infections of various types. The utmost deliberate effort in comprehending the actions of antihistamines within the extent of antimicrobials forms the basis of the investigation. The antimicrobial activity of antihistamines explored by previous researchers helped in determining the minimum inhibitory concentration of cetirizine against 51 strains of bacteria. The antiallergic drug cetirizine showed significant in vitro antimicrobial activity against 51 strains of bacteria belonging to 5 Gram-positive and 4 Gram-negative genera. The minimum inhibitory concentration of the drug determined both by agar dilution and broth dilution method ranged from 200-2000 μg/ml against most of the bacteria tested. Cetirizine was bactericidal in action in vitro on both Gram-positive and Gram-negative bacteria. In vivo experiment with this drug proved that it could offer significant protection to mice challenged with a virulent bacterium Salmonella typhimurium NCTC74. Thus, cetirizine has immense potential to be developed as an antibacterial agent. Therefore the present study would help in corroborating future prospects in disseminating efficacious use of cetirizine in mitigation of microbial infections.
Keywords
Non-antibiotic, antiallergic, antibacterial, bactericidal
The discovery of antimicrobial drugs, often a matter of chance and serendipity till the end of the 19th century, gradually become an exercise of detailed scientific knowledge and wisdom, which resulted in “drug explosion”. Among the antimicrobial agents, largest share belongs to the antibiotics. Enormous use of antibiotics has led not only to emergence of drug resistant bacteria, but also to increasing infections with opportunistic microorganisms. Antimicrobial drugs are the greatest contribution of the 20th century to therapeutics. Their advent changed the outlook of the physician about the power of drugs on diseases. As a class, they are one of the most frequently used as well as misused drugs. So, pharmaceutical industries and research organizations are constantly making an effort to synthesize new antibiotics to combat drug resistance. Extensive studies of various workers detect antimicrobial action in different types of drugs belonging to different pharmacological classes, such as antihistamines like bromodiphenhydramine and diphenhydramine [1], methdilazine [2], promethazine [3], trimeprazine [4], terfenadine [5], tranquilizers like promazine [6], antihypertensives like propranolol [7], methyl dihydroxyphenylalanine (methyl DOPA) [8], dobutamine [9], amlodipine [10], oxyfedrine [11], lacidipine [12], antispasmodics like dicyclomine [13,14], antipsychotics like chlorpromazine [15], fluphenazine [16], thioridazine [17], prochlorperazine [18], flupenthixol [19], antiinflammatory agents like diclofenac [20-24], flurbiprofen [25] and sympathomimetic drug dopamine hydrochloride [26]. Such drugs, having antimicrobial activity in addition to their predesignated pharmacological activity, have been grouped together under the banner of “non-antibiotics” [27].
The present study was performed with cetirizine, an antiallergic drug, to observe its antimicrobial activity by in vitro and in vivo experiments. The antimicrobial activity of this non-antibiotic could be useful in the fight against antimicrobial resistance.
Materials and Methods
Liquid media used in this study were peptone water (PW) containing 1.0% peptone (Oxoid) plus 0.5% AnalaR NaCl, nutrient broth (NB, Oxoid) and Muller Hinton Broth (MHB, Oxoid). Solid media were nutrient agar (NA), prepared by solidifying NB with 2.0% agar (NA, Oxoid), and Muller-Hinton agar (MHA, Oxoid), pH 7.2-7.4.
Total 51 bacterial strains belonging to 5 Gram-positive and 4 Gram-negative genera, comprising of 13 Grampositive and 38 Gram-negative strains were tested (Table 1, Figure 1). Many of the strains were of human origin, identified [28-30] and preserved in freeze dried state. Many of the standard strains like Staphylococcus aureus (ATCC 29157), Bacillus subtilis (ATCC 6633), Micrococcus lutea (ATCC 9341), Vibrio cholerae (ATCC 14033), Escherichia coli (ATCC 25922) and even multidrug resistant (MDR) strains like E. coli (R239), E. coli (R 224), Vibrio cholerae (DN 8), S. aureus (ML 145) and S. aureus (ML 335) were included in the study. All microorganisms were maintained at 4° at slant culture for a maximum of one month and as freeze dried culture for long term preservation [31].
| Name of bacteria | Concentration of cetirizine (μg/ml) | |||||
|---|---|---|---|---|---|---|
| 0 (control) | 200 | 400 | 1000 | 1400 | 2000 | |
| Staphylococcus aureus ML281 | + | + | + | - | - | - |
| Streptococcus feacalis S1 | + | + | + | - | - | - |
| Bacillus subtilis ATCC6633 | + | + | + | - | - | - |
| Micrococcus lutea ATCC9341 | + | + | - | - | - | - |
| Salmonella typhi D1716 | + | - | - | - | - | - |
| Salmonella typhi D642 | + | + | + | + | - | - |
| Salmonella typhi D1604 | + | + | + | + | - | - |
| Vibrio cholerae ATCC14033 | + | + | + | - | - | - |
| Shigella dysenteriae NCTC566/61 | + | + | + | - | - | - |
| Escherichia coli 306 | + | + | + | - | - | - |
| Escherichia coli ATCC25922 | + | + | + | + | + | - |
+ Presence of growth; - absence of growth
Table 1: Preliminary screening of cetirizine as an antimicrobial agent by agar dilution method
Preparation of cetirizine stock solution
Cetirizine used in this study was obtained as pure dry powder of pharmaceutical grade. Specified amount of the drug was accurately weighed and transferred into a suitable sterile volumetric flask and dissolved in sterile distilled water. The flask is covered properly to protect it from light.
Determination of minimum inhibitory concentration (MIC) of cetirizine
MIC of cetirizine was accurately determined with respect to different test bacteria following the standard guideline of agar dilution techniques as described by the clinical laboratories and standard institutes (CLSI 2006). For this purpose, cetirizine was dissolved in sterile distilled water and added to molten Muller Hinton Agar (MHA) at concentration of 0 (control), 200, 400, 1000, 1400 and 2000 μg/ml. Gram-positive bacteria were grown in NB and Gram-negative bacteria in PW for 18 h and bacteria were harvested during the secondary growth phase. A suspension of organism was prepared in 5 ml sterile distilled water. The turbidity of the suspension was adjusted to a 0.5 McFarland standard with a UV/Vis spectrophotometer (Chemito UV 2600 double beam spectrophotometer) at 625 nm, which corresponded to 2.4×108 CFU/ml. the inocula were prepared by further diluting the suspension 1:100 with sterile distilled water in such a manner that a 2 mm (internal diameter) loopful of culture contain 105 CFU. These were spot inoculated on the MHA plates containing increasing concentrations of the drug including the control. The plates were incubated at 37° and examined for the appearance of growth after 24 h (extended up to 72 h, if necessary). The MIC was determined as the concentration of the drug that resulted in no visible growth.
Determination of effect of cetirizine on S. aureus ML 281 and Salmonella typhi 62
To determine the bacteriostatic or bactericidal action of the antihistaminic compound, strains that are sensitive to cetirizine was selected and each of them was grown in 4 ml NB for 18 h. From that 18 h old broth culture, 2 ml was taken and added into another 4 ml of fresh NB. This was incubated at 37° for 2 h so that the bacterial culture could attain logarithmic growth phase. The number of viable cells in the culture was determined by the CFU count technique as described by Miles and Mishra (1938) [34]. At this stage cetirizine was added at a concentration higher than the respective MIC values against the selected sensitive stains. CFU counts from the culture were individually taken after 2 h, 6h and finally after 18 h [32].
In vivo antimicrobial activity
Male albino mice of Swiss strain weighing 18-20 g were taken for in vivo study. Animals were maintained at standard conditions at 21±1° and 50-60% relative humidity with a 14 h photo period. Water and dry pellet diet were given ad libitum. The virulence of the test strain S. typhimurium NCTC74 was exalted by repeated mouse passages and the median lethal dose (MLD or LD50) of the passaged strains was determined. From this the 50×MLD of the strains corresponding to 0.95×109 CFU/mouse suspended in 0.5 ml NB served as the challenge dose [33] for all groups of animals. Reproducibility of the challenged dose was ensured by standardization of its optical density in a colorimeter at 640 nm and determination of the CFU count in NA [34].
To determine the toxicity of cetirizine, 40 mice were taken, 20 of which were injected with 60 μg of drug while the remaining 20 received 100 μg of cetirizine. They were kept under observation for up to 100 h. The protective capacity of cetirizine was judged as follows: two groups of mice, 20 animals per group were kept in separate cages. Group I was intraperitoneally administered 60 μg cetirizine per mouse and group II was given 100 μg of the drug per mouse. After 3 h each group was challenged with 50 MLD of S. typhimurium NCTC 74. A control group of 40 mice was also injected similarly with the same bacterial strain and 0.1 ml sterile saline instead of cetirizine. The protective capacity of the drug was determined by recording the mortality of mice in different groups up to 100 h of treatment and statistically by chi square test.
In another study, 4 groups of 5 mice were used. Group 1 and 3 were injected 100 μg of cetirizine intraperitoneally and each mice of group of 2 and 4 received 0.5 ml of sterile saline instead of drug. After 3 h of treatment, all groups were given 50 MLD challenges of S. typhimurium NCTC74. After 2 h, all mice of group 1 and 2 were sacrificed and their heart blood was collected, livers and spleens were separated aseptically and homogenized in tissue homogenizers. CFU count of individual organs was determined separately. The same procedure was applied to groups 3 and 4, 18 h after challenge. The data obtained were statistically analyzed by student t-test. All the animal experiments were carried out following Institutional Animal Ethical Committee guidelines (955/A/06/CPSEA2006).
Results and Discussion
A primary screening of cetirizine against 7 bacteria belonging to Gram-negative and 4 Gram-positive genera shows satisfactory antimicrobial activity against most of the test bacteria (Table 1). In an elaborate in vitro study, the drug was tested against 42 different strains of bacteria belonging to both Grampositive and Gram-negative genera. Six S. aureus three Bacillus sp., one Enterococcus, Three E. coli, four V. cholera, three Shigells and twenty two Salmonella sp. were used in the elaborate study. Many of them were inhibited at 200-2000 μg/ml concentration; few were also susceptible below 200 μg/ml concentration. The order of sensitivity towards cetirizine was Bacillus sp., Vibiro cholera, S. aureus, Escherichia coli, Shigella sp. But few strains of S. aureus, E. coli, Shigella sp. and Salmonella sp. were not inhibited at test concentration.
The MIC of cetirizine against S. typhi 62 and S. aureus ML281 was 1000 μg/ml; in logarithmic growth phase their CFU count was 5×106 CFU/ml for both of them. At 0 h, 2×MIC of cetirizine of the test organisms was added to each of the culture tubes. Subsequently, when the CFU counts were determined after 2, 6, and 18 h, it was noticed that there was a gradual decrease in the number of viable cells up to 6 h for both bacteria. The decrease in CFU count were 5×1010, 4×1010, 2×107 in the case of S aureus ML281 whereas 5×1010, 1×108, 1×106 in the case of S. typhi 62, respectively. However there were no viable cells found after 18 h, proving the bactericidal property of drug (Figure 2).
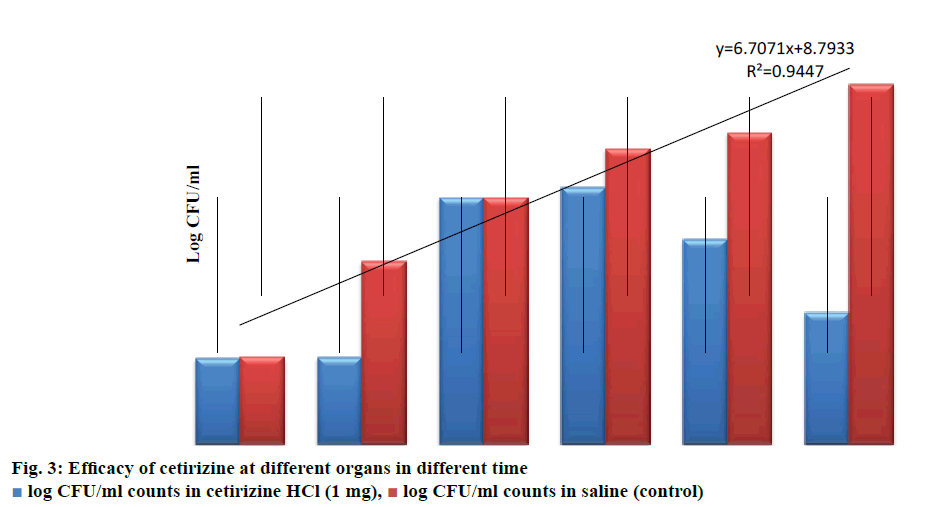
Table 2 shows that in control group 49 out of 60 animals died within 100 h of challenge. No mortality was recorded in those groups that received highest concentration of drug (100 μg/ml). As can be seen in Table 3, by comparing the CFU count in heart blood, liver and spleen at 2 h and 18 h, it is evident that there is no significant increase in viable count in drug treated group even after 18 h, thus clearly indicating the bacteriostatic nature of cetirizine (Figure 3).
| Control Group* | Test Group* | ||
|---|---|---|---|
| Drug injected per mouse(μg) | Mice died out of 60 | Drug injected per mouse (μg) | Mice died out of 20 |
| 0.5 ml sterile saline | 49 | 30 | 13 |
| 60 | 4 | ||
| 100 | 0 | ||
*Received challenged dose of 0.95×109 CFU in 0.5 ml NB of S. typhimurium NCTC74
Table 2: Determination of in vivo protection by cetirizine
| Time of sampling (h) |
Group | No. of mice | Drug conc. per mouse | CFU/ml counts in | ||
|---|---|---|---|---|---|---|
| Heart blood | Liver | Spleen | ||||
| 2 | I | 5 | Cetirizine (1 mg) |
2×102, 2×102, 1×102, 2×102, 2×102 |
5×106, 1×106, 2×106, 3×106, 5×106 |
2.5×105, 2×105, 3.5×105, 1.5×105, 2×105 |
| 2 | II | 5 | Saline (control) | 2×102, 3×102, 1×102, 2×102, 2×102 |
5×106, 1×106, 2×106, 3×106, 5×106 |
1.5×108, 1×108, 1.5×108, 1×108, 2×108 |
| 18 | III | 5 | Cetirizine (1 mg) |
1.5×102, 2×102, 2×102, 2.5×102, 1.5×102 |
5×106, 5×106, 5×106, 4×106, 7×106 | 5×103, 2×103, 3×103, 5×103, 1×103 |
| 18 | IV | 5 | Saline (control) | 5×104, 6×105, 4×104, 6×104, 1×104 | 4×107, 5×107, 5×107, 6×107, 6×107 |
4×109, 5×108, 4×109, 3×109, 4×109 |
Table 3: Efficacy of cetirizine in reducing bacterial counts in challenged mice
The search for antimicrobials has now been extended to a class of compounds named non-antibiotics which are employed for the therapy of noninfectious pathologies and which demonstrate significant antimicrobial activity against some of the most pathogenic infectious agents such as vancomycin resistant or methicillin resistant S. aureus [35] or MDR Mycobacterium tuberculosis [36-38].
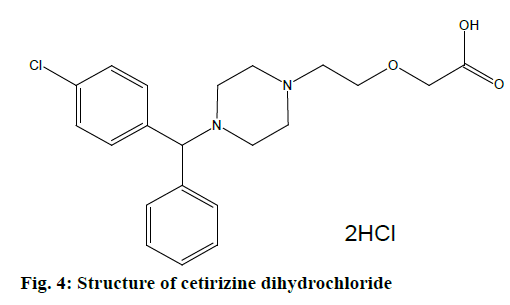
Cetirizine dihydrochloride is an antagonist of histamine, mostly against H1 receptor. It inhibits effect of histamine in H1 receptor of smooth muscle. It also blocks capillary permeability to prevent edema, but as a second generation ethanolamine, it does not cause sedation. Cetirizine HCl is a white powder having a molecular weight of 461.8. Chemically it is (±)-2- [2- [4- [(4-chloropenyl)phenylmethyl]ethoxy] acetic acid dihydrochloride (Figure 4). This drug is sensitive to light and freely soluble in water, partially insoluble in acetone and methyl chloride [39,40].
Cetirizine being a H1 receptor antagonist is used in conditions like upper respiratory allergies, pollinosis, urticarial/atopic dermatitis; also used as adjuvant in seasonal asthma. It is a metabolite of hydroxyzine with marked affinity for peripheral H1 receptor; penetrates brain poorly but subjective drowsiness has been experienced at higher doses. It is not metabolized, does not prolong cardiac action potential or produce arrhythmias when given with erythromycin, ketoconazole. Cetirizine also inhibits release of histamine and cytotoxic mediators from platelets as well as eosinophil chemotaxis during the secondary phase of allergic response. Thus it may benefit allergic disorder by other actions as well. It attains high and longer lasting concentration in skin which may be responsible for superior efficacy in urticarial/atopic dermatitis, as well as once daily dosing although the elimination half-life (t1/2) is 7-10 h [41,42]. In addition to these pharmacological actions, cetirizine has significant activity on several Gram-positive and Gram-negative bacteria in vitro and S. typhimurium in vivo. The study reaffirms the antimicrobial activity of this class of drugs.
Most of the bacteria tested were inhibited within 1000 μg/ml concentration of the drug whereas few Gram-positive and Gram-negative bacteria were killed by the drug at much lower concentration (200-400 μg/ml) of the drug. In an in vitro study, cetirizine is proved as a bactericidal agent, which was performed in a Gram-positive bacteria S. aureus ML281 and a Gram-negative bacteria S. typhi 62. So from the in vitro study it can be concluded that cetirizine produced antimicrobial activity at around 1000 μg/ml concentration but in the in vivo study cetirizine gave significant protection to the challenged mice (with S. typhimurium NCTC74) at 100 μg/ml concentration. In another in vivo study where the viable count of organ homogenates and heart blood were compared to control and drug treated challenged mice, the result were highly significant (P<0.5). Examinations among various classes of pharmacological agents have revealed that in general the tricyclic phenothiazines possess discernible antimicrobial action [43]. Extensive reviews of literature have revealed that antimicrobial properties of several phenothiazines and other antimicrobial agents are due to the presence of aromatic rings [44-46].
Antihistaminic drug cetirizine contains aromatic ring and piperazine ring with halogens. The promising antimicrobial activity of this drug may be attributed to these structural components. Thus, cetirizine stands a chance of being developed as an antimicrobial agent to combat microbial resistance and bacterial infection associated with allergic reactions.
The main limiting factor of non-antibiotics to display their antimicrobial characteristics in mammalian system is that the maximum serum level remains (approximately 1 mg per liter) lower than the concentration required for inhibiting microbial growth. However this level might be sufficient to modify microbial metabolism and act synergistically with certain antibiotics [47]. On the other hands the currently published information describes in vitro and in vivo efficacy in animals. There is very limited clinical information that indicates clinically relevant activity of non-antibiotics in human. In addition, there is a need to take thermodynamics into account in vivo. On the basis of this information new approaches to the infection can be easily designed.
Acknowledgements
The authors wish to thank Ms. Dusap Pharmaceuticals Limited, Kolkata for providing the sample to cetirizine dihydrochloride to carry out the research work. The authors are also thankful to Gupta College of Technological Sciences, India for providing necessary infrastructural facility to carry out the entire research work.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Dastidar SG, Saha PK, Sanyamat B, Chakraborty AN. Antibacterial activity of ambodryl and benadryl. J Appl Bacteriol 1976;41:209-14.
- Chattapadhyay D, Dastidar SG, Chakraborty AN. Antimicrobial property of methdilazine and its synergism with antibiotics and some chemotherapeutic agents. Arzeinmittelforschung 1998;38:869-72.
- Chakraborty AN, Acharya DP, Neogi D, Dastidar SG. Drug interaction of some non-conventional antimicrobial chemotherapeutic agents with special reference to promethazine. Indian J Med Res 1989;89:233-7.
- Dastidar SG, Jairaj J, Mookerjee M, Chakraborty AN. Studies on antimicrobial effect of the antihistaminic phenothiazine trimeprazine tartarate. Acta Microbiol Immunol Hung 1997;44:241-7.
- Perlmutter JI, Forbes LT, Krysan DJ, Ebsworth M, Colgufoun JM, Wang JL, et al. Repurposing the antihistamine terfenadine for antimicrobial activity against S. aureus. J Med Chem 2004;20:8054-62.
- Dash SK, Dastidar SG, Chakraborty P. Antimicrobial activity of promazine hydrochloride. Indian J Exp Biol 1997;15:324-6.
- Manna KK, Dastidar SG. The antihypertensive drug propranolol hydrochloride (carditap): its antimicrobial property. In: Chakraborty AN, Dastidar SG, editors. Proceedings of 6th National Congress IAMM. Calcutta: Image India; 1948. p. 133-7.
- Dastidar SG, Mondal U, Mookerjee M, Chakraborty AN. Antibacterial property of methyl-DOPA and development of cross resistance in m-DOPA mutants. Indian J Med Res 1997;84:142-7.
- Sarkar A, Kumar KA, Dutta NK, Chakraborty P, Dastidar SG. Evaluation of in vitro and in vivo antibacterial activity of dobutamine hydrochloride. Indian J Med Microbiol 2003;21:172-8.
- Kumar KA, Ganguly K, Dutta NK, Mazumdar K, Dastidar SG, Chakraborty AN. Amlodipine: a cardiovascular drug with powerful antimicrobial property. Acta Microbiol Polonica 2003;52:285-92.
- Mazumdar K, Ganguly K, Kumar KA, Dutta NK, Chakraborty AN, Dastidar SG. Antimicrobial potentiality of a new non-antibiotic: The cardiovascular drug oxyfedrine hydrochloride. Res Microbiol 2003;158:1-6.
- Dasgupta A, Dastidar SG. Antimicrobial and antitoxic effects of the cardiovascular drug lacidipine in an animal model. Indian J Med Res 2012;135:913-16.
- Karak P, Kumar KA, Mazumdar K, Mookerjee M, Dastidar SG. Antibacterial potential of an antispasmodic drug dicyclomine hydrochloride. Indian J Med Res 2003;118:192-6.
- Karak P, Kumar KA, Basu LR, Dasgupta A, Ray R, Dastidar SG. Experimental analysis of antimicrobial action of dicyclomine hydrochloride. Biol Pharm Bull 2004;27:2010-17.
- Amaral L, Lorian V. Effects of chlorpromazine on the cell envelope proteins of Escherichia coli. Antimicrob Agents Chemother 1991;35:1923-4.
- Dastidar SG, Chaudhury A, Annadurai S, Roy S, Mukerjee M, Chakraborty AN. In vitro and in vivo antimicrobial action of fluphenazine. J Chemother 1995;7:201-6.
- Radhakrishnan V, Ganguly K, Ganguly M, Dastidar SG, Chakraborty AN. Potentiality of tricyclic compound thioridazine as an effective antibacterial and antiplasmid agent. Indian J Exp Biol 1999;37:671-5.
- Basu LR, Mazumdar K, Dutta NK, Karak P, Dastidar SG. Antibacterial property of the antipsychotic agent prochlorperazine and its synergism with methdilazine. Microbiol Res 2005;160:95-100.
- Jeyaseeli L, Dasgupta A, Dastidar SG, Molnar J, Amaral L. Evidence of significant synergism between antibiotics and the antipsychotic antimicrobial drug fluphenthixol. Eur J Clin Microbiol Infect Dis 2012;31:1243-50.
- Annadurai S, Basu S, Ray S, Dastidar SG, Chakraborty AN. Antimicrobial activity of the antiinflammatory agent diclofenac sodium. Indian J Exp Biol 1998;36:86-90.
- Annadurai S, Guhathakurta A, Sa B, Dastidar SG, Roy R, Chakraborty AN. Experimental studies on synergism between aminoglycosides and the antimicrobial antiinflammatory agent diclofenac sodium. J Chemother 2002;14:47-53.
- Dastidar SG, Annadurai S, Kumar KA, Dutta NK, Chakraborty AN. Evaluation of a synergistic combination between the non-antibiotic microbicides diclofenac and trifluperazine. Int J Antimicrob Agents 2003;2:599-601.
- Dutta NK, Annadurai S, Mazumdar K, Dastidar SG, Kristiansen JE, Molnar J, et al. Potential management of resistant microbial infections with a novel non-antibiotic: the antiinflammatory drug diclofenac sodium. Int J Antimicrob Agents 2007;30:242-9.
- Dutta NK, Mazumdar K, Dastidar SG, Park JH. Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice. Int J Antimicrob Agents 2007;30:336-40.
- Chowdhury B, Adak M, Bose SK. Flurbiprofen, a unique non-steroidal antiinflammatory drug with antimicrobial activity against Trichophyton, Microsporum and Epidermophyton species. Lett Appl Microbiol 2003;37:158-61.
- Maji S, Maji HS, Chakraborty P, Dastidar SG. Potential of dopamine hydrochloride as a novel antimicrobial agent. Int J Biomed Pharm Sci 2010;4:70-5.
- Kristiansen JE, Thomsen VF, Hvidberg EF. The antimicrobial activity of non-antibiotics. Acta Pathol Microbiol Scand 1992;7:19.
- Barrow GI, Feltham RKA. Cowan and Steel’s Manual for the Identification of Medical Bacteria. Cambridge: Cambridge University Press; 1993. p. 50-164.
- Collee JG, Fraser AG, Marmion BP, Simmons A. Mackie and McCartney’s Practical Medical Microbiology. London: Churchill Livingstone (Elsevier); 2006. p. 131-49.
- Collee JG, Fraser AG, Marmion BP, Simmons A. Mackie and McCartney’s Practical Medical Microbiology. London: Churchill Livingstone (Elsevier); 2006. p. 851-52.
- https://clsi.org/standards/products/microbiology/documents/m100/.
- Krogstad DJ, Moellering RC. Combinations of antibiotics, mechanisms of interaction against bacteria. In: Lorian V, Williams X, Wilkins Y, editors. Antibiotic in Laboratory Medicine. Baltimore: Lippincott Williams and Wilkins; 1990. p. 670-5.
- Reed LJ, Meunch H. A simple method for estimating fifty percent end points. Am J Epidemiol 1938;27:493.
- Miles AA, Misra SS. Surface viable count by Miles and Misra method. In: Cruickshank R, Duguid JP, Marmion BP, Swain RHA, editors. Practice of Medical Microbiology. New York: Livingstone Churchill; 1938. p. 307-8.
- Martins M, Bleiss W, Marko A, Ordway D, Viveiros M, Leonardo C, et al. Clinical concentrations of thioridazine enhance the of intracellular methicillin-resistant Staphylococcus aureus: an in vivo, ex vivo and electron microscopy study. In Vivo2004;18:787-94.
- Ordway D, Viveiros M, Leonardo C, Bettencourt R, Almaida J, Martins M, et al. Clinical concentrations of thioridazine kill intracellular multi-drug resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 2003;47:917-22.
- Amaral L, Martins M, Viveiros M. Phenothiazines as anti- multi-drug resistant tubercular agents. Infect Disord Drug Targets 2007;7:257-65.
- Martins M, Dastidar SG, Fanning S, Kristiansen JE, Molnar J, Pages JM, et al. Potential role of non-antibiotics (helper compounds) in the treatment of multidrug resistant Gram negative infections: mechanism for their direct and indirect activities. Int J Antimicrob Ag 2008;31:198-208.
- British Pharmacopoeia. London: HMSO Publication; 2005. p. 418-9.
- Sweetman SC. Martindale: The Complete Drug Reference. 34th ed. London: Pharmaceutical Press; 2005. p. 427.
- Tripathi KD. Essentials of Medical Pharmacology. Hyderabad: Jaypee Publishers; 2013. p. 166-7.
- Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin and their antagonist. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of therapeutics. 12th ed. New York: ΜcGraw Hill; 2011. p. 924.
- Bourlioux P, Moreaux JM, Su WJ, Boureau H. In vitro antimicrobial activity of 18 phenothiazine derivatives, structure-activity relationship. APMIS Suppl 1992;30:40-3.
- Kristiansen JE, Amaral L. The potential management of resistant infections with non-antibiotics. J Antimicrob Chemother 1997;40:319-27.
- Dasgupta A, Jeyaseeli L, Dutta NK, Mazumdar K, Karak P, Dastidar SG, et al. Studies on the antimicrobial potential of the cardiovascular drug lacidipine. In Vivo 2007;21:847-50.
- Dasgupta A, Dastidar SG, Shirataki Y, Motohashi N. Antibacterial activity of artificial phenothiazines and isoflavons from plants. In: Motohashi N, editor. Heterocyclic Compounds. Berlin: Springer-Verlag; 2008. p. 7-132.
- Kalayci S. Antimicrobial properties of various non-antibiotic drugs against microorganisms. J Bioanal Biomed 2016;8:4e142.
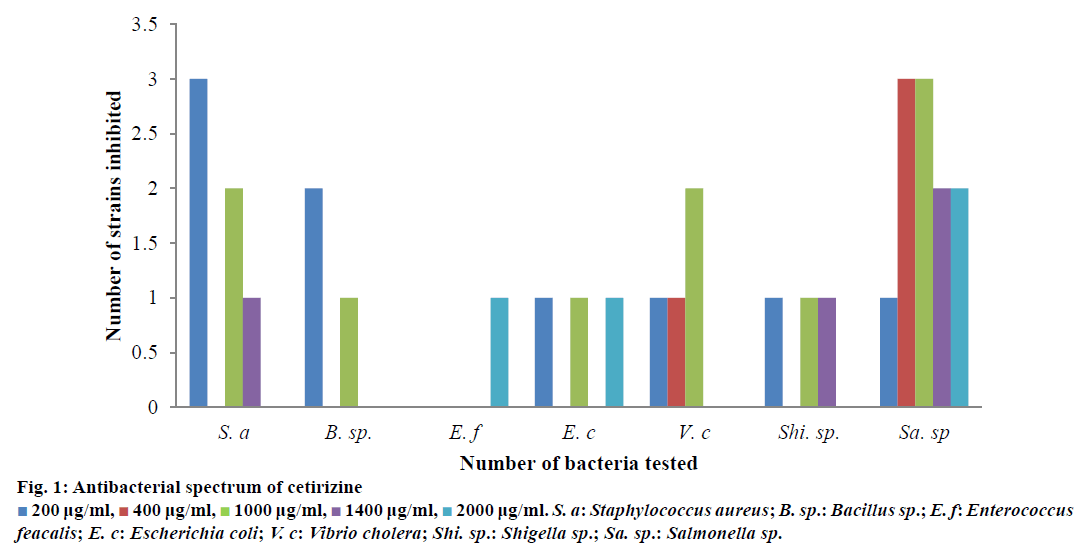
 200 μg/ml,
200 μg/ml, 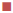 400 μg/ml,
400 μg/ml, 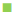 1000 μg/ml,
1000 μg/ml, 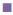 1400 μg/ml,
1400 μg/ml, 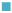 2000 μg/ml. S. a: Staphylococcus aureus; B. sp.: Bacillus sp.; E. f: Enterococcus feacalis; E. c: Escherichia coli; V. c: Vibrio cholera; Shi. sp.: Shigella sp.; Sa. sp.: Salmonella sp.
2000 μg/ml. S. a: Staphylococcus aureus; B. sp.: Bacillus sp.; E. f: Enterococcus feacalis; E. c: Escherichia coli; V. c: Vibrio cholera; Shi. sp.: Shigella sp.; Sa. sp.: Salmonella sp.
 S. aureus ML281,
S. aureus ML281, 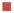 S. typhi 62
S. typhi 62