- *Corresponding Author:
- B. H. M. Mruthyunjayaswamy
Department of Chemistry, Gulbarga University, Kalaburagi, Karnataka 585106, India
E-mail: drbhiremath25@gmail.com
| Date of Received | 02 May 2020 |
| Date of Revision | 07 August 2021 |
| Date of Acceptance | 31 January 2022 |
| Indian J Pharm Sci 2022;84(1):115-120 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Two simple, sensitive and precise spectrophotometric methods for the assay of cefixime in either pure form or in its pharmaceutical dosage form are described. The method I is based on the reaction of salicylaldehyde with cefixime resulting in a yellow coloured product, absorbs at λmax 425 nm. The second method describes the reaction between the diazotized drug and N-(1-naphthyl)ethylenediamine dihydrochloride to yield a purple coloured product with λmax at 567 nm. The reaction conditions were optimized to get maximum colour intensity. The absorbance was found to extend linearly with increasing the concentration of cefixime the systems obeyed the Beer’s law within the range of 2-10 μg/ml and 5-25 μg/ml for salicylaldehyde and N-(1-naphthyl)ethylenediamine dihydrochloride methods. Common excipients used as additives in pharmaceutical dosage don’t interfere within the proposed analytical methods. The products are stable for over 6 h and 10 h respectively. The proposed methods are simple, sensitive, accurate and suitable for quality control uses.
Keywords
Cefixime, salicylaldehyde, N-(1-naphthyl)ethylenediamine dihydrochloride, pharmaceutical dosage form, validation
Cefixime (CFX) is designated and chemically known as 7-(Z)- [2-(2-aminothiazol-4-yl)-2- carboxymethoxyimino)acetamido]-3-cephem-4- carboxylic acid trihydrate [1,2]. CFX is an antibiotic and third generation cephalosporin and it is extremely stable within the presence of beta-lactamase enzymes. CFX is employed within the treatment of the subsequent infections caused by susceptible strains of the designated microorganism; uncomplicated urinary tract infections caused by Escherichia coli and Proteus mirabilis, otitis media caused by Haemophilus influenza (beta- lactamase positive and negative strains), Moraxella catarrhalis most of which are beta-lactamase positive and Streptococcus pyogenes, pharyngitis and tonsillitis caused by Streptococcus pyogenes, acute bronchitis and acute exacerbations of continual bronchitis caused by Streptococcus pneumonia and Haemophilus influenzae and uncomplicated gonorrhea caused by Neisseria gonorrhea.
Literature survey reveals that only a few methods like spectrophotometric [3-6], High Performance Liquid Chromatography (HPLC) [7-10], High Performance Thin Layer Chromatography (HPTLC) [11,12], Liquid Chromatography-Mass Spectrometry (LC-MS) [13,14], high performance capillary electrophoresis [15,16] and spectrofluorimetric methods [17-19], are available for the analysis of CFX. In continuation of our research on spectrophotometric determination of organic compounds of pharmaceutical importance, this communication reports two spectrophotometric methods for the determination of CFX in either pure form or in pharmaceutical dosage forms. Both regents are used for the primary time in CFX analysis.
The simplicity of these methods is that the reagent utilized in both the methods is well available and therefore the chemistry of the reagent is already well established. The reaction involved these reagents are easy, rapid and sensitive within the ranges of determinations compared with other established methods. These methods involve the formation of highly coloured species that are stable for 6 h and 10 h respectively, which makes it easier for the determination. In continuation of our research work on drug analysis [20-23], we hereby report two simple and sensitive spectrophotometric methods for the determination of CFX in either pure form or in pharmaceutical dosage forms.
Materials and Methods
Apparatus:
An ELICO Model SL-164 double beam, Ultraviolet (UV)-visible spectrophotometer with 1.0 cm matched quartz cells was used for absorption measurements.
Materials:
Drug (Gift sample from Alkem Laboratories Limited, India), salicylaldehyde (SLD) (S.d. Fine Chem., India) and N-(1-naphthyl)ethylenediamine dihydrochloride (NEDA) (S.d. Fine Chem., India) were used. All other chemicals and solvents were of analytical reagent grade.
Standard solutions:
CFX (pure or dosage) (100 mg) was accurately weighed and dissolved in 20 ml of 0.1 N Hydrochloric Acid (HCl) solution and then the resulting solution was transferred to a regular 100 ml volumetric flask. The ultimate volume was made upto the mark with demineralised water. The ultimate concentration was brought upto 100 µg/ml with demineralised water. A 0.5 % alcoholic solution of SLD and 0.5 % aqueous solution of NEDA were freshly prepared.
Analytical procedure:
In method I, aliquots of the working standard solution (0.2-1.0 ml) of CFX (100 µg/ml) were transferred into a series of 10 ml calibrated flask. To each, 1.0 ml of 0.5 % alcoholic solution of SLD was added. The solutions were swirled and heated to 60° to 70° for 10 min. After cooling, the level was made upto the mark with demineralised water. The absorbance was measured at 425 nm against corresponding reagent blank and calibration graph was constructed.
In method II, aliquots of the working standard solution (0.5-2.5 ml) CFX (100 µg/ml) were transferred into 10 ml calibrated flasks, 0.2 ml of concentrated HCl was added, cooled in an ice bath and 1.0 ml of 0.5 % sodium nitrite solution was added. The solutions were cooled to 0° and 0.5 ml of 0.5 % ammonium sulfamate solution was added and stirred for 5 min. Then 1.0 ml of 0.5 % NEDA solution was added and made upto the mark with demineralised water. The solutions were mixed thoroughly and the absorbance was measured at 567 nm against reagent blank and calibration graph was constructed.
Assay of the drug in commercial dosage forms:
10 to 20 tablets looking on the content per tablet were weighed and mixed thoroughly. An amount of the powder corresponding to 100 mg of the active component was weighed into a 100 ml volumetric flask, about 60 ml of 0.1 N HCl solution was added and shaken thoroughly for about 20 min, the level was increased to the mark with demineralised water, shaken and filtered using paper. For spectrophotometric determination, the filtrate was diluted sequentially to induce 100 µg/ml for drug.
Results and Discussion
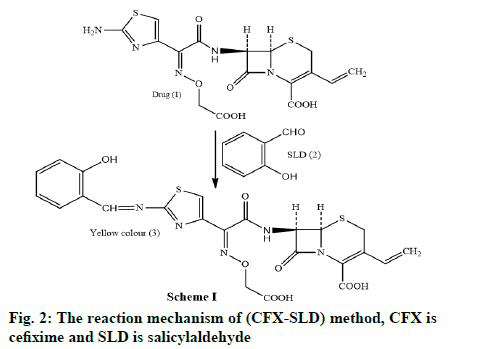
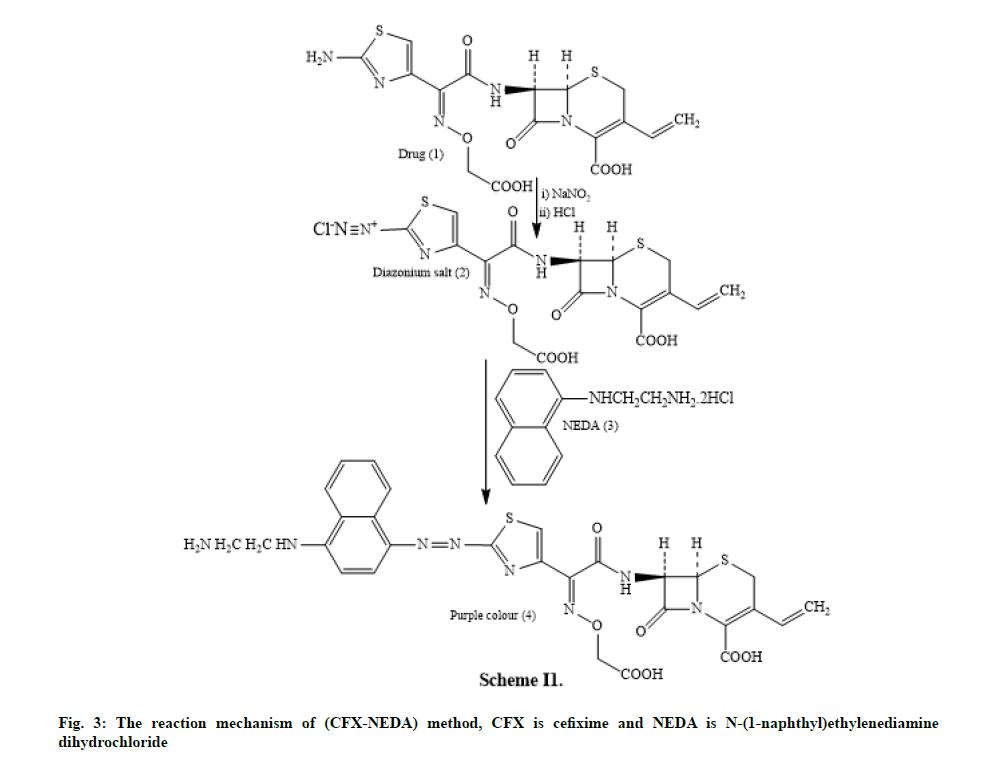
In the method I, the drug undergoes condensation with alcoholic SLD to form Schiff’s base and concerning the method II, the drug undergoes diazotization followed by coupling with the NEDA in aqueous medium.
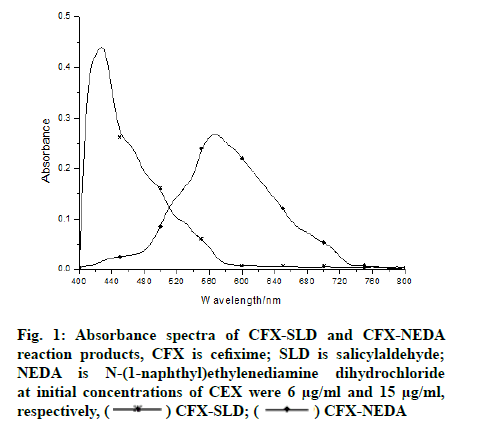
The absorbance spectra of the yellow coloured product (CFX-SLD) with wavelength of maximum absorbance (λmax) 425 nm and of the purple coloured product (CFXNEDA) with λmax 567 nm are shown in fig. 1. The above mentioned blanks have practically negligible absorption in both the methods.
In the method I, it was found that 0.2-1.0 ml of 0.5 % alcoholic SLD solution was necessary to achieve maximum colour intensity. In the method II, it was found that 0.2-1.0 ml of concentrated HCl, 0.5- 2.5 ml of 0.5 % sodium nitrite, 0.5-2.5 ml of 0.5 % ammonium sulfamate and 0.5-2.5 ml of 0.5 % aqueous NEDA solutions were necessary for the occurrence of maximum colour intensity. The surplus of nitrite might be removed by the addition of 0.5 ml of 0.5 % ammonium sulfamate which has no consequence on the color intensity of the product formed. Just in case of NEDA as a coupling agent, dilution of the coloured solution with different solvents like water, methanol, ethanol, carboxylic acid and acetonitrile are tested. However, dilution with water gave maximum intensity and stability of colour. The central composite experimental design has been used to screen the effect of both the reagents in the proposed methods.
For the method I and II, Beer’s law was obeyed over the concentration range of 2-10 µg/ml and 5-25 µg/ ml respectively. The proposed procedures were validated by determining various optical parameters which are presented in Table 1. The linearity, slope and intercepts are calculated using the regression equation y=ax+b, where ‘y’ represents optical density, ‘x’ the concentration of the drug in µg/ml and ‘a’ and ‘b’ represents slope and intercepts, respectively. Precision and accuracy of the proposed methods were tested by effecting the determination of eight replicates of pure and commercial sample of the drug, whose concentration lie within Beer’s law range. The values of Standard Deviation (SD), Relative Standard Deviation (RSD) and range of error at a 95 % confidence limit level were calculated.
There was no change in λmax when the smallest amount concentration of the analytes is determined, for the calculation of Limit of Detection (LOD). Limit of Quantification (LOQ) was found to be 3.3 times that of LOD, which is in accordance with Thumb’s rule. The experiment for the proposed methods was conducted by the second analyte on different days and the consequences produced, justified the ruggedness of the proposed method. These methods are applied to pharmaceutical dosage forms and recovery studies are made. The optical characteristics and precision data for these two methods recommended are presented in Table 1.
| Parameter/characteristics | Method I | Method II |
|---|---|---|
| Colour | Yellow | Purple |
| λmax (nm) | 425 | 567 |
| Stability (h) | 6 | 10 |
| Beer’s law range (μg/ml) (C) | 2-10 | 5-25 |
| LOD (μg/ml) | 0.28 | 0.26 |
| LOQ (μg/ml ) | 0.92 | 0.86 |
| Molar absorptivity (l/mol/cm) | 3.584×104 | 7.951×103 |
| Sandell’s sensitivity (μg/cm2 ) | 0.015 | 0.057 |
| Regression equation (Y)a | ||
| Slope (a) | 2.2×10-2 | 2.3×10-2 |
| Intercept (b) | 6.2×10-2 | 1.5×10-3 |
| Correlation coefficient (r) | 0.9996 | 0.9999 |
| RSD (%)b | 0.32 | 0.53 |
| % Range of error’sb | ±0.0018 | ±0.0017 |
Note: RSD is relative standard deviation; aY=ax+b, where x is the concentration in μg/ml; beight replicates
Table 1: Parameters For The Spectrophotometric Determination of Drug
In method I the drug containing aromatic amine undergoes condensation with alcoholic SLD yields a yellow coloured Schiff’s base product scheme I (fig. 2). Method II includes the diazotization of the drug to make diazonium salt which on coupling with NEDA yields a purple dye. The reaction mechanism (CFX- NEDA) method is revealed in scheme II (fig. 3). The coloured products were found to be stable for 6 h and 10 h respectively at room temperature. Reproducible results were obtained within the temperature range of 20°-70°.
A detailed study of the interference of assorted concomitant substances on the determination of the drugs was made. For method I, 6 µg/ml CFX was chosen to test interference. In method II, 15 µg/ml CFX was utilized for the study of interference. Prior to the addition of reagents, a known amount of the interfering substance was added and thus the reaction was conducted for both the methods. The extent of interference by various excipients is studied for both methods and is tabulated in Table 2. It is observed that both the methods delivered excellent results for the determination of pure CFX within the presence of excipients which don’t interfere in both the methods. An inaccuracy of 2.0 % in the absorbance reading was considered allowable.
| Material | Amount (mg) | % Recovery of drug±RSDb | |
|---|---|---|---|
| Method I | Method II | ||
| Magnesium stearate | 20 | 99.68±0.28 | 99.79±0.16 |
| Lactose | 20 | 99.79±0.12 | 99.86±0.24 |
| Dextrose | 20 | 99.77±0.14 | 99.68±0.13 |
| Starch | 20 | 99.78±0.12 | 99.76±0.24 |
| Gum acacia | 20 | 99.68±0.24 | 99.81±0.19 |
| Talc | 20 | 99.72±0.32 | 99.68±0.22 |
| Carboxy methyl cellulose | 20 | 99.89±0.15 | 99.70±0.41 |
| Sodium alginate | 20 | 99.62±0.21 | 99.67±0.36 |
Note: RSD is relative standard deviation; aMethod I, 6 μg/ml of drug taken; Method II, 15 μg/ml of drug taken; bAverage of five determinations
Table 2: Determination of CFX in The Presence of Excipient and Other Substancesa
The developed methods were applied to the purity of active component in commercially obtainable tablets and thus the results are signified in the Table 3. The identical batch tablets were also analyzed by the official method [6]. A statistical analysis of the results using the Student’s t-test and F-test showed no significant differences with relevance to the accuracy and precision. The variety of statistical methods may be used to compare three or more sets of data; however, the most commonly used method is an Analysis of Variance (ANOVA) in t-test and F-test which can clearly describe the validation of the proposed spectroscopic methods statistically. The reliability and accuracy of the methods were further confirmed by recovery studies by the standard-addition method.
| Preparationsa | Label claim mg/Tablet | Foundb (recovery±SD) | Students t-valuec | F-valued | ||||
|---|---|---|---|---|---|---|---|---|
| Method I | Method II | Reference method | I | II | I | II | ||
| Taxim-O (1) | 50 | 99.78±0.36 | 99.55±0.41 | 99.86±0.24 | 0.52 | 1.85 | 2.25 | 2.91 |
| Mahacef (2) | 100 | 99.75±0.42 | 99.68±0.46 | 99.94±0.46 | 0.86 | 1.31 | 1.20 | 2.06 |
| Ominicef-O (3) | 200 | 99.66±0.42 | 99.63±0.21 | 99.78±0.32 | 0.64 | 1.11 | 1.72 | 2.32 |
Note: SD is standard deviation; aMarketed by (1) Alkem, (2) Mankind and (3) Aristo; bMean value of eight determinations; cTabulated value at 95 % confidence level is 2.365; dTabulated value at 95 % confidence level is 3.79
Table 3: Results of An Assay of CFX in Pharmaceutical Dosage
To a set and known quantity of the drug during a tablet solution (pre-analysed), pure CFX was added at three dissimilar levels which was found by the proposed methods. Each level was repeated 3 times using three different market dosages. The percent recoveries of the added pure drug are given in Table 4, which specifies normally encountered tablets, excipients didn’t interfere with the determination by the proposed methods. The proposed methods use eco-friendly and need economical chemicals and rarely employ organic solvents.
| Dosagea | Method I | Method II | ||||||
|---|---|---|---|---|---|---|---|---|
| Amount of CFX in dosage/mg |
Amount of drug added/mg |
Total amount found/mg | % Recovery of pure drugb |
Amount of CFX in Dosage/mg |
Amount of drug added/mg |
Total amountfound/mg | % Recovery of pure drugb | |
| 1 | 3.95 | 3 | 6.94 | 99.67 | 4.95 | 4 | 8.94 | 99.75 |
| 3.95 | 6 | 10.0 | 100.83 | 4.95 | 8 | 12.98 | 100.37 | |
| 3.95 | 9 | 12.92 | 99.67 | 4.95 | 12 | 16.98 | 100.25 | |
| 2 | 4.99 | 3 | 8.0 | 99.33 | 6.02 | 4 | 10.05 | 100.75 |
| 4.99 | 6 | 10.98 | 99.83 | 6.02 | 8 | 14.0 | 99.75 | |
| 4.99 | 9 | 14.0 | 100.11 | 6.02 | 12 | 18.0 | 99.83 | |
| 3 | 5.97 | 3 | 9.0 | 101.0 | 8.98 | 4 | 12.97 | 99.75 |
| 5.97 | 6 | 11.95 | 99.66 | 8.98 | 8 | 16.99 | 100.12 | |
| 5.97 | 9 | 14.96 | 99.88 | 8.98 | 12 | 20.97 | 99.92 | |
Note: CFX is cefixime; aBranded by (1) Taxim-O (50 mg); (2) Mahacef (100 mg) and (3) Ominicef-O (200 mg); bAverage of three determinations
Table 4: Results of Recovery Studies By The Standard-Addition Technique
CFX is determined by a different kind of technique, the methods illustrated here are simple, sensitive, convenient and don’t entail special working conditions. The statistical parameters and the recovery study data evidently indicate the reproducibility and accuracy of the new experimental design approach of spectrophotometric method for the determination of CFX. These methods may possibly be considered for the determination of CFX in routine pharmaceutical laboratories.
Acknowledgements:
The authors are grateful to the authority of Department of Chemistry, Gulbarga University, Kalaburagi, for providing research facilities.
Conflict of interests:
The authors declared no conflict of interest.
References
- Sweetman SC. Martindale: The Complete Drug Reference. 38th ed. London: Pharmaceutical press; 2014.
- Niel MJO. The Merck index: An encyclopedia of chemicals, drugs and biologicals. 13th ed. USA: Whitehouse Station, Merck & Co., Inc; 2001. p. 160.
- Mali AD. Zero, first, second order derivative and area under curve spectrophotometric methods for determination of cefixime trihydrate in pharmaceutical formulation. Int J Pharm Pharm Sci 2015;7(6):321-5.
- Ramadan AA, Mandil HA, Dahhan MA. UV-VIS Spectrophotometric study for determination of cefixime in pure form and in pharmaceuticals through complexation with Cu (II) using acetate-NaOH buffer in water:methanol. Int J Pharm Pharm Sci 2013;5(1):428-33.
- Ramadan AA, Mandil H, Dahhan M. Spectrophotometric determination of cefixime in pure form and in Syrian pharmaceuticals through complexation with Cu (II). Asian J Chem 2013;25(6):3457-62.
- Ahmad NR, Omar FK. Spectrophotometric determination of cefixime through Schiff’s base system using vanillin reagents inpharmaceutical preparations. Irq Nat J Chem 2013;49:38-46.
- Khandagle KS, Gandhi SV, Deshpande PB, Gaikwad NV. A simple and sensitive RP-HPLC method for simultaneous estimation of cefixime and ofloxacin in combined tablet dosage form. Int J Pharm Pharm Sci 2011;3(1):46-8.
- Arshad HM, Gauhar S, Bano R, Muhammad IN. Development of HPLC-UV method for analysis of cefixime in raw materials and in capsule. Jordan J Pharm Sci 2009;2(1):53-65.
- Khan A, Iqbal Z, Khan MI, Javed K, Khan A, Ahmad L, et al. Simultaneous determination of cefdinir and cefixime in human plasma by RP-HPLC/UV detection method: Method development, optimization, validation and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879(24):2423-9.
[Crossref] [Google Scholar] [PubMed]
- Adam EH, Saeed AE, Barakat IE. Development and validation of a high performance liquid chromatography method for determination of cefixime trihydrate and its degraded products formed under stress condition of UV light. Int J Pharm Sci Res 2012;3(2):469-73.
- Pawar SJ, Kale AP, Amrutkar MP, Jagade JJ, Pore NS, Bhosale AV. HPTLC estimation of cefixime and cloxacillin in tablet dosage form. Asian J Res Chem 2010;3(2):299-301.
- Rao J, Sethy K, Yadav S. Validated HPTLC method for simultaneous quantitation of cefixime and ofloxacin in bulk drug and in pharmaceutical formulation. Int J Compr Pharm 2011;2(4):1-5.
- Meng F, Chen X, Zeng Y, Zhong D. Sensitive liquid chromatography-tandem mass spectrometry method for the determination of cefixime in human plasma: Application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2005;819(2):277-82.
[Crossref] [Google Scholar] [PubMed]
- Talebpour Z, Pourabdollahi H, Rafati H, Abdollahpour A, Bashour Y, Aboul-Enein HY. Determination of cefixime by a validated stability-indicating HPLC method and identification of its related substances by LC-MS/MS studies. Sci Pharm 2013;81(2):493-504.
[Crossref] [Google Scholar] [PubMed]
- Honda S, Taga A, Kakehi K, Koda S, Okamoto Y. Determination of cefixime and its metabolises by high-performance capillary electrophoresis. J Chromatogr A 1992;590(2):364-8.
[Crossref] [Google Scholar] [PubMed]
- Alnajjar AO. Simultaneous determination of ofloxacin and cefixime in tablet formulation using capillary electrophoresis. J Liq Chromatogr Relat Technol 2013;36(19):2687-97.
- Shah J, Jan MR, Shah S. Spectrofluorimetric method for determination and validation of cefixime in pharmaceutical preparations through derivatization with 2-cyanoacetamide. J Fluoresc 2011;21(2):579-85.
[Crossref] [Google Scholar] [PubMed]
- Manzoori JL, Amjadi M, Soltani N, Jouyban A. Spectrofluorimetric determination of cefixime using terbium-danofloxacin probe. Iran J Basic Med Sci 2014;17(4):256-62.
[Google Scholar] [PubMed]
- Abdollahi A, Tabrizi AB. Determination of some cephalosporins in pharmaceutical formulations by a simple and sensitive spectrofluorimetric method. Pharm Sci 2016;22(1):28-34.
- Hiremath B, Mruthyunjayaswamy BH. Development and validation of spectrophotometric methods for the determination of cefetamet in pharmaceutical dosage forms. Chin J Chem 2007;25(12):1827-31.
- Hiremath B, Mruthyunjayaswamy BHM. Spectrophotometric determination of a phenolic β-lactum antibiotic in pure forms and in their pharmaceutical formulations. J Indian Council Chem 2007;24(1):85-9.
- Hiremath B, Mruthyunjayaswamy BH. Development and validation of spectrophotometric methods for determination of ceftazidime in pharmaceutical dosage forms. Acta Pharm 2008;58(3):275-85.
[Crossref] [Google Scholar] [PubMed]
- Hiremath B, Mruthyunjayaswamy BH. Development and validation of a high-performance liquid chromatographic determination of ceftriaxone sodium and its application to drug quality control. Anal Lett 2009;42(14):2180-91.