- *Corresponding Author:
- Xiaozhen Zhu
Department of General surgery, Pingyang Hospital affiliated to Wenzhou Medical University, Whenzhou 325000, China
E-mail: ZXZ1632021@163.com
| Date of Received | 28 February 2022 |
| Date of Revision | 31 March 2022 |
| Date of Acceptance | 12 May 2023 |
| Indian J Pharm Sci 2023;85(3):761-768 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To probe the functions of amygdalin on thyroid cancer cells and further analyze whether its mechanism is related to regulating circWHSC1 and miR-195-5p expression. The relative levels of circWHSC1 and miR-195-5p in cells were analyzed by reverse transcription-quantitative polymerase chain reaction. Effects of amygdalin on thyroid cancer cell lines were calculated. Dual luciferase report assay was applied to ensure the targeting between circWHSC1 and miR-195-5p. The level of circWHSC1 was increased, while the level of miR-195-5p was declined in thyroid cancer tissues. Amygdalin treatment dose-dependently reduced cell viability, suppressed cell invasion, migration and colony formation abilities. Amygdalin treatment reduced the level of circWHSC1, as well as increased the level of miR-195-5p. CircWHSC1 directly bound to miR-195-5p. CircWHSC1 inhibition notably restrained cell growth and mobility in thyroid cancer. Moreover, circWHSC1 overexpression abated the suppressing functions of amygdalin on thyroid cancer cell proliferative, migratory and invasive abilities. Amygdalin inhibited cell proliferative, migratory and invasive abilities in thyroid cancer via circWHSC1/miR-195-5p axis.
Keywords
Amygdalin, thyroid cancer, circWHSC1, miR-195-5p, proliferation
Thyroid Cancer (TC) is the most common endocrine malignancy, about 80 % of which is Papillary TC (PTC), and its incidence rate is on the rise[1]. Although the outcome of this cancer has improved after specific treatments such as surgery, radioiodine and thyrotropin suppression therapy, there is still a risk of tumor recurrence and metastasis[2]. Thus, exploring more targeted agents or effective strategies is of significant clinic value for improving the life quality of patients with TC.
In recent years, modern research have manifested the important role of Traditional Chinese Medicine (TCM) in disease treatment, as the indispensable part of TCM, Herbal Medicine (HM) possesses inhibitory functions on the oncogenic phenotypes of tumor cells, and possesses the characteristics of multiple targets, wide therapeutic pathways and small side effects, showing significant advantages in cancer therapy[3,4]. Amygdalin is one of HM extracted from Rosaceae nuclei that possesses antioxidant, immunomodulatory, antibacterial, anti-fibrosis effects and antitussive effects. Meanwhile, amygdalin is also discovered to have anti-tumor activity on solid tumors, including lung, kidney and bladder cancers via modulating cell survival, immune function and cytotoxicity[5,6], whereas, the functions of amygdalin in TC has not been reported.
Circular Ribonucleic Acids (circRNAs) and micro Ribonucleic Acids (miRNAs) are two types of noncoding RNAs that have been proposed to be responsible for the development of cancers by acting as promoters or inhibitors[7]. Ovarian cancer showed high expression of circWHSC1 and deficiency of circWHSC1 induced apoptosis and suppressed mobility in tumor cells via miR-1182 and miR-145 in a target manner[8]. Whereas, the functions of circWHSC1 in the TC has not been reported. In the preliminary bioinformatics analysis, miR-195-5p was showed to have complementary sequences on circWHSC1. The cancer-suppressing role of miR-195-5p in lung cancer[9] and colorectal cancer[10,11] has been identified. Besides, miR-195-5p weakened cell malignant growth in TC by targeting Lipase member H[12]. CircRNA NRIP1 declined miR-195-5p level in a target manner to weaken the growth of TC tumors[13]. Whether circWHSC1 is involved in regulating TC tumorigenesis via miR-195-5p remains unclear. In addition, preliminary experiments showed the changes of circWHSC1 and miR-195-5p levels in TC cells after amygdalin treatment, thus, we speculated amygdalin might affect TC development via modulating circWHSC1 and miR-195-5p. Here, this study aims to probe the function of amygdalin on TC cell proliferative, invasive and migratory capacities, and further explore its molecular mechanism through circWHSC1 and miR-195-5p.
Materials and Methods
Cell culture and treatment:
Nthy-ori 3-1 normal cells and TC cell lines SW579 (American Type Culture Collection (ATCC), Manassas, VA, USA) cultured in Dulbecco's Modified Eagle Medium (ATCC) with 1 % penicillin/streptomycin and 10 % Fetal Bovine Serum (FBS) (Solarbio, Shanghai, China) at 37° with 5 % CO2. Amygdalin was purchased from China Institute for Food and Drug Inspection and Verification (Beijing, China). SW579 cells were exposed to increasing dose of amygdalin (0, 10 mg/l, 20 mg/l, 40 mg/l) for 24 h for functional analysis.
Cell transfection:
The specific siRNA targeting circWHSC1 (si-circWHSC1), circWHSC1 overexpressing vector (circWHSC1), miR-195-5p mimic (miR-195-5p) and the matching control (si-NC, pcDNA-circWHSC1 or miR-NC) were obtained from Genepharma (Shanghai, China). The transient transfection was implemented adopting Lipofectamine 2000. After transfection, cells were subjected amygdalin stimulation for subsequent analysis.
RNA extraction and quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR):
The isolation of total RNAs was performed using the Trizol reagent (Beyotime, Shanghai, China), then complementary DNAs (cDNAs) were generated by reverse transcription, and qRT-PCR amplification using cDNAs as template was conducted with SYBR Premix Ex Taq (Takara, Kusatsu, Japan). The content of circWHSC1 and miR-195-5p was calculated through the Cycle threshold value.
CCK-8 assay:
SW579 cells collected from different groups were seeded in 96-well plates, and then 1 µl of CCK-8 solution (Beyotime) was added into per well for 2 h-incubation. The absorbance value at 450 nm was tested with a spectrophotometer.
Colony formation assay:
SW579 cells collected from different groups were maintained in 6-well plates with completed medium for 14 d. Then the colonies were counted after being stained with 0.1 % crystal violet (Beyotime).
Transwell assay:
After indicated treatment, SW579 cells (about 5×105) with serum-free medium were plated into the upper chamber of Transwell filters (Beyotime) without (for migration) or with (for invasion) Matrigel-coated membrane. Medium with 10 % FBS was filled into the bottom chamber. 48 h later, crystal violet staining was conducted and the migrated and invaded cells were counted under a microscope.
Western blotting:
The total protein was extracted by Radioimmunoprecipitation Assay (RIPA) buffer (Beyotime) and then separated by 10 % SDS-PAGE gel. After being transferred into PVDF membrane[14], the membrane was enclosed with 5 % skim milk for 2 h at 37°, followed by incubating with E-cadherin (1:800), N-cadherin (1:500) and GAPDH (1:800) at 4° for 12 h. The membrane was then probed with the second antibodies at 37° for 1 h and then each band was analyzed by electrochemiluminescence system.
Dual-luciferase reporter assay:
The theoretical binding sequence of miR-195-5p on circWHSC1 and their mutated sequence were cloned into pmirGLO vectors (GenePharma) to establish Wild-Type (WT) and Mutated (MUT) luciferase reporter vector (WT-circWHSC1 or MUT-circWHSC1). The 200 ng recombinant vectors together with 50 nM miR-195-5p or miR-NC were transfected into SW579 cells. 48 h later, luciferase activity was detected.
Statistical analysis:
The data were expressed as (x?±s). The comparison between groups was executed using SPSS 21.0 statistical software with the t-test or analysis of variance. p<0.05 indicated statistically significant.
Results and Discussion
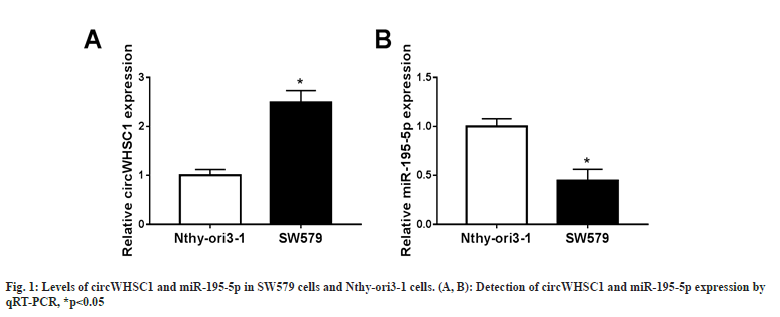
SW579 cell lines showed higher circWHSC1 levels relative to normal Nthy-ori3-1 cells (fig. 1A), while miR-195-5p content was significantly lower in SW579 cells in comparison to Nthy-ori3-1 cells (fig. 1B). Therefore, deregulated circWHSC1 or miR-195-5p might be related to TC cell dysfunction.
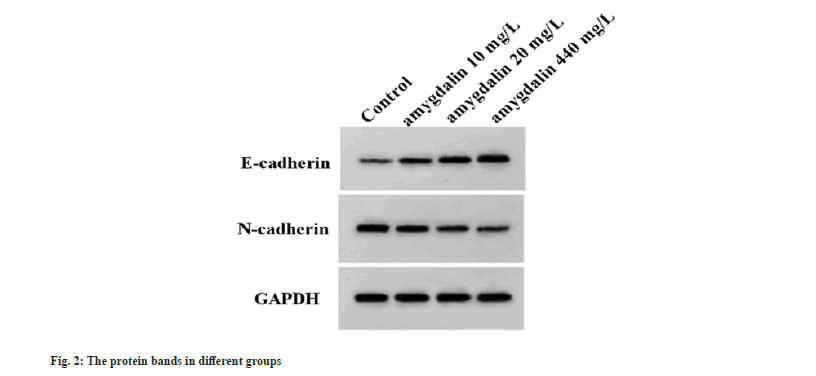
SW579 cells were exposed to increasing dose of amygdalin. Functionally, it was found that with the increasing dose of amygdalin, the OD values and the number of colonies were significantly reduced in SW579 cells (Table 1). Besides that, migrated and invaded cells were also decreased after amygdalin introduction by way of dose-dependent manner (Table 1). Moreover, the EMT-related protein was detected and we found amygdalin dose-dependently elevated E-cadherin content but decreased N-cadherin content in SW579 cells (Table 1 and fig. 2).
| Group | OD values | The number of colonies | The number of migrated cells | The number of invaded cells | E-cadherin | N-cadherin |
|---|---|---|---|---|---|---|
| Control | 1.05±0.07 | 125.00±5.35 | 188.67±5.73 | 152.67±4.78 | 0.16±0.01 | 0.63±0.05 |
| Amygdalin 10 mg/l | 0.86±0.06a | 104.33±4.92a | 161.67±4.92a | 131.33±3.36a | 0.34±0.03a | 0.44±0.04a |
| Amygdalin 20 mg/l | 0.61±0.04ab | 81.67±2.87ab | 126.33±3.68ab | 102.00±2.94ab | 0.51±0.05ab | 0.28±0.02ab |
| Amygdalin 40 mg/l | 0.40±0.02abc | 58.67±2.49abc | 78.67±2.49abc | 60.33±3.30abc | 0.73±0.04abc | 0.16±0.01abc |
| F | 92.419 | 146.162 | 353.27 | 357.492 | 139.059 | 108.239 |
| p | 0 | 0 | 0 | 0 | 0 | 0 |
Note: Relative to the control group, ap<0.05; Relative to amygdalin 10 mg/l group, bp<0.05 and Relative to amygdalin 20 mg/l group, cp<0.05
Table 1: The effects of Amygdalin on TC Cell Proliferation, Migration and Invasion.
Next, the effect of amygdalin on circWHSC1 and miR-195-5p level was studied. As displayed in Table 2, amygdalin dose-dependently decreased circWHSC1 content and increased miR-195-5p content in SW579 cells.
| Group | circWHSC1 | miR-195-5p |
|---|---|---|
| Control | 1.00±0.00 | 1.00±0.00 |
| Amygdalin 10 mg/l | 0.83±0.04a | 1.43±0.09a |
| Amygdalin 20 mg/l | 0.55±0.04ab | 2.40±0.12ab |
| Amygdalin 40 mg/l | 0.24±0.02abc | 3.83±0.16abc |
| F | 369.786 | 392.915 |
| P | 0.000 | 0.000 |
Note: Relative to the control group, ap<0.05; Relative to amygdalin 10 mg/l group, bp<0.05 and Relative to amygdalin 20 mg/l group, cP<0.05.
Table 2: The effects of Amygdalin on circWHSC1 and miR-195-5p expression level in TC Cells.
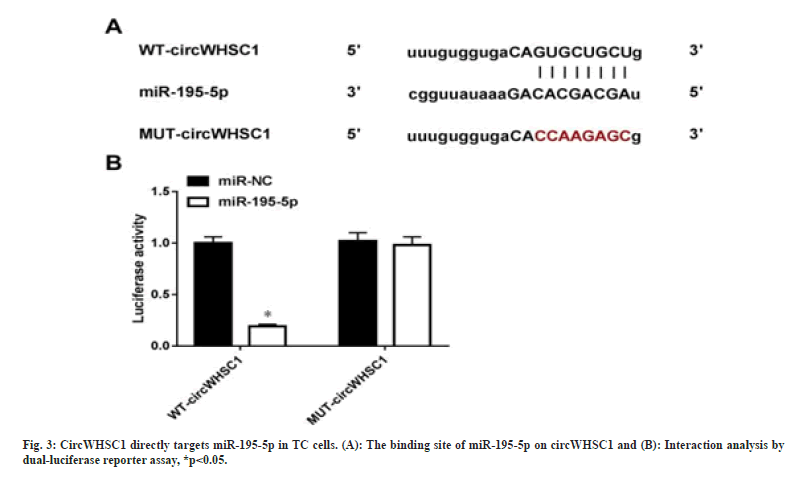
As exhibited in fig. 3A, Starbase predicted that miR-195-5p possesses binding site on circWHSC1. Then it was discovered that miR-195-5p overexpression markedly declined the luciferase activity in WT-circWHSC1 group, but not affected the mutated one in SW579 cells (fig. 3A). In all, miR-195-5p was directly targeted by circWHSC1 in TC cells.
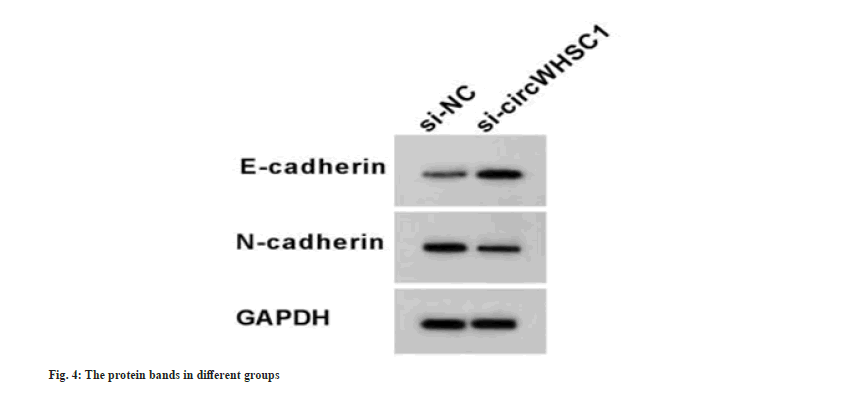
Compared with the si-NC transfection, knockdown of circWHSC1 by si-circWHSC1 markedly declined circWHSC1 level and increased miR-195-5p level in SW579 cells (Table 3). Functionally, circWHSC1 silencing suppressed SW579 cell proliferation, evidenced by decreased optical density values and colonies number, and restrained cell migration and invasion (Table 3). Meanwhile, si-circWHSC1 introduction led to the elevation of E-cadherin and decline of N-cadherin in SW579 cells (Table 3 and fig. 4). Thus, circWHSC1 deficiency weakened TC cell growth and mobility.
| Group | circWHSC1 | miR-195-5p | OD values | The number of colonies | The number of migrated cells | The number of invaded cells | E-cadherin | N-cadherin |
|---|---|---|---|---|---|---|---|---|
| si-NC | 1.00±0.00 | 1.00±0.00 | 1.07±0.04 | 25.33±6.80 | 189.33±6.94 | 152.67±7.93 | 0.17±0.02 | 0.62±0.07 |
| si-circWHSC1 | 0.33±0.02a | 3.15±0.09a | 0.51±0.03a | 68.33±3.40a | 91.00±4.55a | 81.67±2.87a | 0.65±0.03a | 0.22±0.02a |
| t | 58.024 | 41.377 | 19.399 | 9.796 | 20.523 | 14.582 | 23.058 | 80.891 |
| p | 0 | 0 | 0 | 0.001 | 0 | 0 | 0 | 0 |
Note: Relative to si-NC group, ap<0.05
Table 3: The effects of circWHSC1 on TC Cell Proliferation, Migration and Invasion.
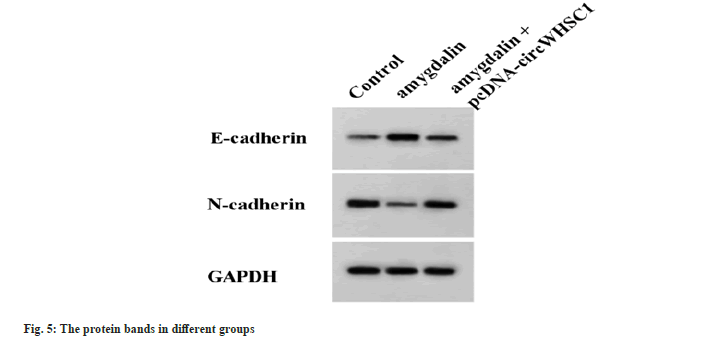
Relative to the control group, amygdalin treatment suppressed SW579 cell proliferation, migration and invasiveness, while the suppressing effects caused by amygdalin were abolished by following circWHSC1 overexpression (Table 4 and fig. 5).
| Group | circWHSC1 | miR-195-5p | OD values | The number of colonies | The number of migrated cells | The number of invaded cells | E-cadherin | N-cadherin |
|---|---|---|---|---|---|---|---|---|
| Control | 1.00±0.00 | 1.00±0.00 | 1.07±0.06 | 124.33±6.55 | 188.33±7.72 | 153.33±7.59 | 0.17±0.02 | 0.63±0.06 |
| Amygdalin | 0.24±0.02a | 3.83±0.15a | 0.41±0.02a | 58.67±2.62a | 77.00±3.27a | 59.33±4.03a | 0.74±0.04a | 0.15±0.01a |
| Amygdalin+pcDNA-circWHSC1 | 0.86±0.05b | 1.27±0.08b | 0.92±0.05b | 107.33±3.86b | 171.67±5.31b | 139.67±3.86b | 0.26±0.03b | 0.53±0.04b |
| F | 507.722 | 759.623 | 165.739 | 161.63 | 329.499 | 261.589 | 291.414 | 108.906 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to the control group, ap<0.05 and Relative to amygdalin group, bP <0.05
Table 4: circWHSC1 overexpression reverses the anticancer effects of Amygdalin on TC cells.
Currently, TC has posed a remarkable threat to the lives and health of women worldwide, and it is particularly urgent to explore its potential progression mechanisms and find new and effective treatment strategies for TC. Amygdalin has been reported to play an anticancer function in various tumors. For instance, Lee et al.[15] manifested that amygdalin was able to impair cell adhesion and induce cell apoptosis in breast cancer. Makarevi? et al.[16] suggested that amygdalin could decrease the expression of CDK2 and cyclin A, thereby suppressing the survival of bladder cancer cells. In addition, amygdalin was demonstrated to have anti-migration and anti-invasion effects in lung cancer to impede cancer metastasis[17]. In the current work, we also found the anticancer functions of amygdalin in TC. Amygdalin treatment was discovered to suppress TC cell proliferative ability, and restrain cell migratory and invasive abilities in a dose-dependent manner. E-cadherin and N-cadherin are Epithelial Mesenchymal Transition (EMT)-related markers, and they are complete membrane proteins that form adhesion connections. During EMT, N-cadherin is up-regulated and E-cadherin is down-regulated, EMT process has been shown to be critical in invasion and metastasis[18]. It has been reported that N-cadherin level in TC was significantly elevated, the ectopic expression of N-cadherin induced growth and invasiveness of TC cells, while knockdown of it showed contrary effects[19]. Besides, loss of E-cadherin was remarkably associated with lymph node metastasis in TC[20]. Here, this study also found that amygdalin treatment dose-dependently up-regulated E-cadherin content and down-regulate N-cadherin content in TC cells, which was consistent with the inhibitory effects of amygdalin on invasion and migration.
It has been proposed that circRNA is widely involved in multiple cellular processes of cancer progression via targeting the miRNA, and is considered to be an important molecular target for cancer treatment[21,22]. CircWHSC1 is a functional circRNA. It exhibited stronger tumorigenicity to nude mice, and stimulated cell growth and mobility in endometrial cancer[23]. CircWHSC1 deficiency restrained the ability to grow and metastasize in liver cancer cells, and hindered tumorigenesis in nude mice by affecting the level of miR-142-3p[24]. In our study, TC cell lines exhibited increased circWHSC1 level and declined miR-195-5p content in TC cell lines, moreover, miR-195-5p level was boosted while circWHSC1 level was declined following amygdalin treatment in TC cells, suggesting that the anticancer mechanism of amygdalin may be in connection with miR-195-5p and circWHSC1. Further function experiments showed that circWHSC1 miss weakened ability to grow and metastasize in TC cells. In addition, this study also confirmed that miR-195-5p was targeted by circWHSC1. Early study exhibited miR-195-5p could impair cell growth and EMT by declining YAP1 in cervical cancer[25]. MiR-195-5p interacted with circ_0020850 to play anti-proliferation activity in lung adenocarcinoma[26]. In addition, the oncogenic behaviors of TC cells were discovered to be arrested by miR-195-5p[27-29]. Thus, circWHSC1 could regulate TC progression via miR-195-5p. Meanwhile, rescue assay showed that the suppressing effects caused by amygdalin on the oncogenic behaviors of TC cells were weakened through the overexpression of circWHSC1, indicating that the anticancer effect of amygdalin in TC is at least related to regulating the circWHSC1 and miR-195-5p.
In all, this work confirmed that amygdalin performed anticancer effects in TC by decreasing cancer cell proliferative, invasive and migratory capacities. Moreover, the mechanism of amygdalin was achieved via the inhibition of circWHSC1/miR-195-5p axis. These data preliminarily revealed the antitumor activity and mechanism of amygdalin on thyroid cancer, and provided theoretical basis for the development of amygdalin in thyroid cancer therapy.
Conflict of interests:
The authors declared no conflict of interests.
References
- James BC, Timsina L, Graham R, Angelos P, Haggstrom DA. Changes in total thyroidectomy versus thyroid lobectomy for papillary thyroid cancer during the past 15 years. Surgery 2019;166(1):41-7.
[Crossref] [Google Scholar] [PubMed]
- Ruiz EM, Niu T, Zerfaoui M, Kunnimalaiyaan M, Friedlander PL, Abdel-Mageed AB, et al. A novel gene panel for prediction of lymph-node metastasis and recurrence in patients with thyroid cancer. Surgery 2020;167(1):73-9.
[Crossref] [Google Scholar] [PubMed]
- Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med 2019;8(5):1958-75.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T. Salidroside ameliorates Parkinson's disease by inhibiting NLRP3-dependent pyroptosis. Aging (Albany NY) 2020;12(10):9405-26.
[Crossref] [Google Scholar] [PubMed]
- Makarevi? J, Tsaur I, Juengel E, Borgmann H, Nelson K, Thomas C, et al. Amygdalin delays cell cycle progression and blocks growth of prostate cancer cells in vitro. Life Sci 2016;147:137-42.
[Crossref] [Google Scholar] [PubMed]
- Alwan AM, Rokaya D, Kathayat G, Afshari JT. Onco-immunity and therapeutic application of amygdalin: A review. J Oral Biol Craniofac Res 2023;13(2):155-63.
[Crossref] [Google Scholar] [PubMed]
- Xu BB, Zheng ED, Sun HY, Huang Y, Zheng L, Lan QL, et al. Comprehensive analysis of circular RNA-associated competing endogenous RNA networks and immune infiltration in gastric cancer. Transpl Immunol 2023;77:101793.
[Crossref] [Google Scholar] [PubMed]
- Zong ZH, Du YP, Guan X, Chen S, Zhao Y. CircWHSC1 promotes ovarian cancer progression by regulating MUC1 and hTERT through sponging miR-145 and miR-1182. J Exp Clin Cancer Res 2019;38(1):437.
[Crossref] [Google Scholar] [PubMed]
- Luo J, Pan J, Jin Y, Li M, Chen M. MiR-195-5p inhibits proliferation and induces apoptosis of non-small cell lung cancer cells by targeting CEP55. Onco Targets Ther 2019;12:11465-74.
[Crossref] [Google Scholar] [PubMed]
- Feng C, Zhang L, Sun Y, Li X, Zhan L, Lou Y, et al. GDPD5, a target of miR-195-5p, is associated with metastasis and chemoresistance in colorectal cancer. Biomed Pharmacother 2018;101:945-52.
[Crossref] [Google Scholar] [PubMed]
- Jin Y, Wang M, Hu H, Huang Q, Chen Y, Wang G. Overcoming stemness and chemoresistance in colorectal cancer through miR-195-5p-modulated inhibition of notch signaling. Int J Biol Macromol 2018;117:445-53.
- Gui X, Li Y, Zhang X, Su K, Cao W. Circ_LDLR promoted the development of papillary thyroid carcinoma via regulating miR-195-5p/LIPH axis. Cancer Cell Int 2020;20:241.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhu L, Fu L, Han M, Li Y, Meng Z, et al. CircRNA NRIP1 promotes papillary thyroid carcinoma progression by sponging mir-195-5p and modulating the P38 MAPK and JAK/STAT pathways. Diagn Pathol 2021;16(1):93.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Wu J, Li Y, Jiang Y, Wang L, Chen Y, et al. Circ_0000527 promotes the progression of retinoblastoma by regulating miR-646/LRP6 axis. Cancer Cell Int 2020;20:301.
[Crossref] [Google Scholar] [PubMed]
- Lee HM, Moon A. Amygdalin regulates apoptosis and adhesion in Hs578T triple-negative breast cancer cells. Biomol Ther 2016;24(1):62-6.
[Crossref] [Google Scholar] [PubMed]
- Makarevi? J, Rutz J, Juengel E, Kaulfuss S, Reiter M, Tsaur I, et al. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PloS One 2014;9(8):e105590.
[Crossref] [Google Scholar] [PubMed]
- Qian L, Xie B, Wang Y, Qian J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int J Clin Exp Pathol 2015;8(5):5363.
[Google Scholar] [PubMed]
- Debnath P, Huirem RS, Dutta P, Palchaudhuri S. Epithelial–mesenchymal transition and its transcription factors. Biosci Rep 2022;42(1).
[Crossref] [Google Scholar] [PubMed]
- Da C, Wu K, Yue C, Bai P, Wang R, Wang G, et al. N-cadherin promotes thyroid tumorigenesis through modulating major signaling pathways. Oncotarget 2017;8(5):8131.
[Crossref] [Google Scholar] [PubMed]
- Zhou C, Yang C, Chong D. E-cadherin expression is associated with susceptibility and clinicopathological characteristics of thyroid cancer: A PRISMA-compliant meta-analysis. Medicine 2019;98(30):e16187.
[Crossref] [Google Scholar] [PubMed]
- Lin D, Lin X, He T, Xie G. Gambogic acid inhibits the progression of gastric cancer via circRNA_ASAP2/miR-33a-5p/CDK7 axis. Cancer Manag Res 2020;12:9221.
[Crossref] [Google Scholar] [PubMed]
- Verduci L, Strano S, Yarden Y, Blandino G. The circ RNA–micro RNA code: Emerging implications for cancer diagnosis and treatment. Mol Oncol 2019;13(4):669-80.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Chen S, Zong ZH, Guan X, Zhao Y. CircRNA WHSC1 targets the miR?646/NPM1 pathway to promote the development of endometrial cancer. J Cell Mol Med 2020;24(12):6898-907.
[Crossref] [Google Scholar] [PubMed]
- Lyu P, Zhai Z, Hao Z, Zhang H, He J. CircWHSC1 serves as an oncogene to promote hepatocellular carcinoma progression. Eur J Clin Invest 2021;51(6):e13487.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Zhou Y, Ning YE, Gu H, Tong Y, Wang N. MiR-195-5p inhibits malignant progression of cervical cancer by targeting YAP1. Onco Targets Ther 2020;13:931-44.
[Crossref] [Google Scholar] [PubMed]
- Xin T, Li S, Zhang Y, Kamali X, Liu H, Jia T. circRNA Hsa_circ_0020850 silence represses the development of lung adenocarcinoma via regulating miR-195-5p/IRS2 axis. Cancer Manag Res 2020;12:10679.
[Crossref] [Google Scholar] [PubMed]
- Du P, Liu F, Liu Y, Shao M, Li X, Qin G. Linc00210 enhances the malignancy of thyroid cancer cells by modulating miR?195?5p/IGF1R/Akt axis. J Cell Physiol 2020;235(2):1001-12.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Zhang L, Chen W, Yuan F, Yang Z, Liu S, et al. miR-195-5p regulates cell proliferation, apoptosis, and invasion of thyroid cancer by targeting telomerase reverse transcriptase. Bioengineered 2021;12(1):6201-9.
[Crossref] [Google Scholar] [PubMed]
- Liu D, Li P, Wang X, Wang W. hsa-miR-195-5p inhibits cell proliferation of human thyroid carcinoma cells via modulation of p21/cyclin D1 axis. Transl Cancer Res 2020;9(9):5190.
[Crossref] [Google Scholar] [PubMed]