- *Corresponding Author:
- Archana Negi Sah
Department of Pharmaceutical Sciences,
Faculty of Technology,
Bhimtal Campus,
Kumaun University,
Nainital,
Uttarakhand 263136,
India
E-mail: drarchanansah@gmail.com
| Date of Received | 11 November 2019 |
| Date of Revision | 19 November 2021 |
| Date of Acceptance | 09 March 2022 |
| Indian J Pharm Sci 2022;84(2):287-299 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Study demonstrates the antiurolithiatic potential of the three important plant species of the western Himalayan region viz. Ficus palmata fruits, Raphanus sativus leaves and Nyctanthes arbor-tristis leaves in vitro. Nucleation, growth and aggregation assays along with microscopic analysis of calcium oxalate crystals was employed to investigate the antilithic effect of the hydroethanolic extracts of Ficus palmata fruits, Raphanus sativus leaves and Nyctanthes arbor-tristis leaves on crystallization events of calcium oxalate stone formation. Fourier-transform infrared spectroscopy and high performance liquid chromatography analysis was employed for characterizing the phytoconstituents present in the extracts. All the three plant extracts produced inhibition of nucleation, growth and aggregation, and reduction of number and size of calcium oxalate crystals. A favorable morphological transformation of calcium oxalate crystals was also witnessed in the presence of the hydroethanolic extracts of Raphanus sativus and Nyctanthes arbortristis. Phytochemical investigation of the extracts revealed the presence of saponins, tannins, flavonoids and polyphenolic compounds while Fourier transform-infrared spectroscopy and high performance liquid chromatography analysis further substantiated the presence of polyphenolic compounds which are known to be involved in producing the anticrystallization effect of the tested extracts. Study confirmed that Ficus palmata fruits, Raphanus sativus leaves and Nyctanthes arbor-tristis leaves possess significant anticrystallization activity against calcium oxalate crystals which may translate to brilliant antiurolithiatic activity based on the effect of these extracts on various phases of urinary stone formation as witnessed in the present study.
Keywords
Catechin, caffeic acid, fourier transform-infrared spectroscopy, high performance liquid chromatography, nucleation, aggregation, urolithiasis, urinary stones
Urolithiasis or kidney stone disease is usually described as a disease that results from disruption of equilibrium between promoters and inhibitors of stone formation [1]. It is an enigmatic disease with complex etiology which is persistently on rise and has emerged as a common yet excruciating affliction that accounts for frequent emergency department visits [2]. Urolithiasis is known to currently afflict approximately 12 % inhabitants of the World’s industrialized nations [3,4]. Due to global warming, further 10 % hike in this statistics is anticipated within next 50 y [5]. As per the reports from the National Health and Nutrition Examination Survey (NHANES), prevalence of nephrolithiasis in United States of America (USA) increased from 5.2 % to 8.8 % since the year 1998 to 2010. Similarly, prevalence of urolithiasis in Germany increased from 4 % in 1979 to 4.7 % in 2001 and in China prevalence rate increased from 1.5 % to 4 % from the year 1989 to 2000 [6]. India falls in the Afro-Asian stone belt of high stone prevalence [7]. In India, urolithiasis contributes to numerous cases of chronic renal diseases and renal failures [8].
Calcium Oxalate (CaOx) stones have been the most studied stone types for the last few decade [9]. The reason being, CaOx is the most predominant chemical entity of urinary stones [10] and is the most recurrent type of all the stones [11]. CaOx also presents the most challenging class of stone disease due to their majorly idiopathic nature [12] and complex etiology [13]. Due to this, available treatment options have not been found to be completely effective so far [14]. Therefore, the current study appertains to mitigating CaOx urolithiasis which is the most prevalent of all urinary stone diseases [2].
Herbs are more like a panacea for vivid range of afflictions and ailments. Plants continue to be a vital part of therapeutics and medicine worldwide. An era of renaissance of phytotherapy is being witnessed wherein plants and phytoconstituents are increasingly grabbing interest as a potential source of drug discovery and development [15]. Phytoconstituents like caffeine have been reported to prevent urolithiasis by inducing translocation of crystal binding annexin A1 proteins from the apical surface of the renal tubular cells to the cytoplasm [16]. Other phytoconstituents like catechin [17], resveratrol [18], rutin, curcumin [19], quercetin and hyperoside [20] have shown promising outcomes as antiurolithiatic in animal models.
The Himalayan region is a treasure of biodiversity and harbors immensely rich flora and fauna. The present study addresses Ficus palmata Forsk. (F. palmata) (Moraceae), Raphanus sativus L.(R. sativus) (Cruciferae) and Nyctanthes arbor-tristis L. (N. arbor- tristis) (Oleaceae) of the western Himalayan region for their antiurolithiatic activity in vitro. F. palmata or Wild Himalayan Fig (Bedu) is an underexplored plant of high medicinal value [21]. Contrarily, R. sativus or radish and N. arbor-tristis or Night Jasmine (Harsingar) has been reported for their therapeutic potential in wide range of diseases and ailments. F. palmata possesses reported nephroprotective activity [22] and N. arbor- tristis have been shown to possess diuretic activity [23]. Roots of R. sativus have been reported to be antilithic [24] and diuretic [25]. Antioxidant property reported in all the three plants [26-28] is a unifying feature and is of utmost significance in context to the present study. Despite of the indications in various nephrological disorders, none of the selected plant parts have been evaluated for their plausible antiurolithiatic activity. Hence, this study was conducted to demonstrate the in vitro antilithic potential of the fruits of F. palmata, leaves of R. sativus and N. arbor-tristis. Predilection for the use of fruits of F. palmata [29], leaves of R. sativus [30] and leaves of N. arbor-tristis [31] is based on the tradi30tional use of these specified plant parts in urinary stone treatment or as diuretics.
Materials and Methods
Plant collection:
The plant samples of R. sativus L. and F. palmata Forsk. were collected from Bhimtal region and N. arbor-tristis L. were collected from Haldwani region of Uttarakhand situated in the foothills of Himalaya. Plant specimens were authenticated from Botanical Survey of India (BSI), Dehradun and a voucher specimen of each with accession number 116594, 116591 and 12611, respectively was also deposited in the herbarium of BSI. Leaves of R. sativus were collected in the month of April while ripe fruits of F. palmata and leaves of N. arbor-tristis were collected in the month of July.
Extract preparation:
The collected plant parts were dried in shade, powdered and were subjected to extraction by cold maceration in 70 % v/v ethanol for 96 h. Marc was separated using grade 1 Whatman filter paper and the extracts were dried in a rotary evaporator under reduced temperature and pressure [32-34]. Extractive yield of R. sativus Leaf Extract (RSLE) obtained was 14.15 % and that of F. palmata Fruit Extract (FPFE) and N. arbor-tristis Leaf Extract (NALE) was 17.403 % and 10.62 %, respectively.
Preliminary phytochemical evaluation:
Prepared extracts were subjected to qualitative phytochemical screening for the presence of phytoconstituents like carbohydrates, proteins, steroids, alkaloids, glycosides, saponins, flavonoids, tannins and phenolic substances [35,36].
Quantification of total phenolic and flavonoid content:
Total Phenolic Content (TPC) of the extracts was determined by Folin-Ciocalteau method as described by Singleton et al. with minor modifications [37]. Briefly, to 0.5 ml of 1 mg/ml extract, 2 ml Folin-Ciocalteau reagent (10 %) was added followed by the addition of 2 ml sodium carbonate solution (7.5 %). The reaction mixture was allowed to stand at room temperature for 1 h and the absorbance was recorded at 760 nm. A standard calibration curve for gallic acid (5-100 mg/l) was plotted and TPC of each extract was expressed as mg of Gallic Acid Equivalent (GAE) per g of dry weight of extract [38].
Total Flavonoid Content (TFC) of the extracts was determined by Aluminium Chloride (AlCl )colorimetric method. To 0.5 ml of 1 mg/ml extract 1.5 ml ethanol, 0.1 ml AlCl3 solution and 0.1 ml potassium acetate solution was added followed by the addition of 3 ml distilled water. The reaction mixture was allowed to stand at room temperature for 1 h and the absorbance was measured at 415 nm. A standard calibration curve for quercetin (5-100 mg/l) was plotted and TFC of each extract was expressed as mg of Quercetin Equivalent (QE) per g of dry weight of extract [38].
Fourier Transform-Infrared (FT-IR) characterization of the extracts:
The three extracts viz. NALE, FPFE and RSLE were characterized using PerkinElmer FT-IR by attenuated total reflectance technique [39].
High Performance Liquid Chromatography (HPLC) analysis of the extracts:
HPLC analysis of FPFE, NALE and RSLE was performed by Reverse Phase High Performance Liquid Chromatography (RP-HPLC) in Agilent 1200 series HPLC system. HPLC was performed using Agilent Zorbax Eclipse plus RP-C18 column (4.6×250 mm; particle size 5 mm) at 45° with a solvent flow rate of 1.0 ml/min and injection volume of 20 μl at 254 nm wavelength. Mobile phase consisted of water (eluent A) and acetonitrile (eluent B). The following gradient program was used for the separation of analytes: 0-5 min, 5 % B; 5-20 min, 10 % B; 20-25 min, 100 % B; 25-30 min 100 % B; 30-35 min, 5 % B; 35-40 min, 5 % B.
Nucleation assay:
Nucleation assay of CaOx crystallization was used to evaluate the effect of the extracts on CaOx crystal formation. For this, 100-1000 µg/ml concentrations of the extracts were prepared in distilled water. To 1 ml of each concentration of the extract was added with 3 ml of 5 mmol/l Calcium Chloride (CaCl2) solution and 3 ml of 7.5 mmol/l Sodium Oxalate solution (Na2C2O4), both prepared in a Tris (Hydroxymethyl) Aminomethane Hydrochloride (Tris-HCl) (0.05 mol/l) and Sodium Chloride (NaCl) (0.15 mol/l) buffer at pH 6.5. Final solutions were vortexed and incubated at 37° for 30 min and their Optical Density (OD) was measured using a Shimadzu UV-1601 Ultraviolet-Visible (UV-Vis) spectrophotometer at 620 nm wavelength. The extent of nucleation in the presence and absence of the extracts was determined and expressed as percent (%) inhibition of nucleation by incorporating the recorded OD in formula: % Inhibition=(1-ODTest/ODControl)×100 [40]. Cystone (Himalaya Herbal Healthcare), a polyherbal formulation commonly employed as a standard substance in various antilithiatic studies [41,42] was also evaluated in similar set up that served as standard.
Microscopic characterization:
CaOx crystals formed in metastable solutions prepared by the addition of CaCl2 solution and Na2C2O4 solution were viewed using a Leica DM 2500 LED microscope and their number, size and morphology was determined [41].
Aggregation assay:
To determine the effect of the extracts on aggregation of CaOx crystals, seed CaOx crystals were prepared by mixing 50 mmol/l each of CaCl2 and Na2C2O4 solution. Crystal slurry thus produced was dried and 0.8 mg/ml solution of CaOx crystals was prepared in a Tris-HCl (0.05 mol/l) and NaCl (0.15 mol/l) buffer (pH 6.5). To 3 ml of CaOx solution was added 1 ml of varying concentrations (100-1000 μg/ml) of the extracts and Cystone and the OD of the test samples and standard was read on UV-Vis spectrophotometer at 620 nm wavelength after 30 min incubation at 37°. Percent inhibition of aggregation was calculated using formula: % Inhibition= (1-ODTest/ODControl)×100 [40].
Growth assay:
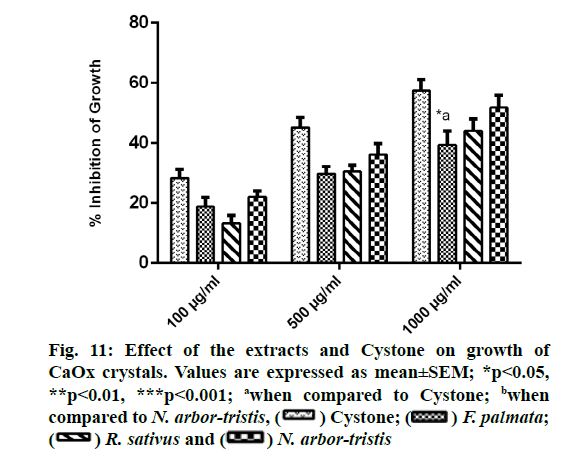
Effect of the extracts on CaOx crystal growth was determined by means of oxalate depletion assay. For this, to 1.5 ml buffer system containing 10 mM Tris- HCl and 90 mM NaCl (pH 7.4) was added 1 ml each of CaCl2 solution (4 mM) and Na2C2O4 solution (4 mM). Finally, 30 μl of 1.5 mg/ml CaOx crystal slurry prepared in 50 mM sodium acetate buffer (pH 5.7) was added and depletion of oxalate from the solution was recorded over a period of 600 s at 214 nm wavelength on a UVVis spectrophotometer as a measure of CaOx crystal growth. Growth inhibitory effect of the extracts and Cystone was then recorded at varying concentrations (100 μg/ml, 500 μg/ml and 1000 μg/ml) by addition of 1 ml solution of the extracts. Difference in the rate of oxalate depletion before and after the addition of the extracts was taken into account and expressed as percent inhibition of growth by using formula: % Inhibition=(1-ODTest/ODControl)×100 [43].
Statistical analysis:
Quantitative data was expressed as mean±Standard Error of Mean (SEM). Statistical computations and analysis of the data were performed using one way Analysis of Variance (ANOVA) followed by Tukey- Kramer’s multiple comparison test with the help of GraphPad Prism 6 software, p values less than 0.05 were considered statistically significant.
Results and Discussion
Presence of carbohydrates, steroids, saponins, flavonoids, tannins and phenols was confirmed in all the three extracts while alkaloids and glycosides were also detected in RSLE in addition to the other phytoconstituents.
Substantial amount of phenols and flavonoids were confirmed in all the evaluated plant extracts. Among the three extracts, highest concentration of phenolic compounds was recorded for NALE followed by RALE and FPFE. Highest amount of flavonoid content was present in RSLE followed by NALE and FPFE (Table 1).
| Extract | TPC (mg GAE/g extract) | TFC (mg QE/g extract) |
|---|---|---|
| FPFE | 2.069±0.008 | 1.413±0.018 |
| RSLE | 3.359±0.014 | 2.72±0.022 |
| NALE | 3.504±0.137 | 2.036±0.016 |
Note: All data are presented as mean±SEM (n=3); GAE: Gallic acid equivalent; QE: Quercetin equivalent
Table 1: Total Phenolic and Flavonoid Content of the Hydroethanolic Extracts
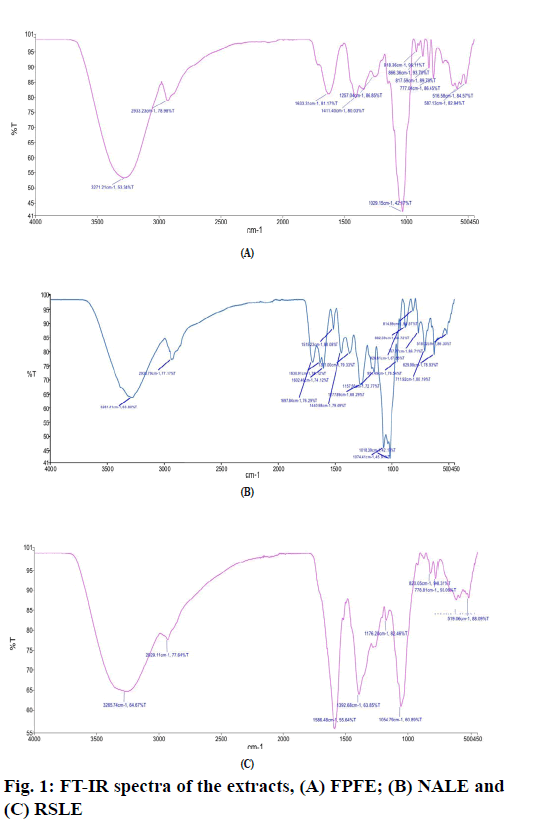
The FT-IR spectrum of FPFE (fig. 1A) showed a characteristic broad O-H stretching band at 3271.21 cm-1, C-H stretching band at 2933.23 cm-1, a band at 1633.31 cm-1 due to C=C stretching vibration of alkene or probably due to –NHCO amide group. A sharp peak at 1029.15 cm-1 may be due to the C-O stretching.
FT-IR spectrum of NALE (fig. 1B) showed a broad peak at 3281.41 cm-1 corresponding to the O-H stretching band, C-H stretching at 2935.79 cm-1, strong peak at 1697.84 cm-1 due to amide C=O stretching -NH stretching vibration at 1440.98 cm-1, -CH3 at 1371 cm-1 and a peak at 1277.89 cm-1 probably due to C-O of polyols. Two sharp peaks at 1074.41 cm-1 and 1018.39 cm-1 may be due to C-O stretching vibrations and secondary alcohols or due to C-N stretch of amines. A band at 1515.23 cm-1 probably be due to C=C stretching of aromatic ring. Moreover, peaks at 3281.41, 2935.79, 1697.84, 1630.91, 1515.23, 1440.98, 1277.89, 1074.41, 951.49, 882.59, 814.89 and 767.27 cm-1 correspond to that of arbortristoside B has been found to be similar to that reported by Purushothaman et al. [44].
FT-IR spectrum of RSLE (fig. 1C) showed the presence of O-H stretching band at 3265.74 cm-1, C-H stretching at 2929.11 cm-1 and -NH bending and -CH bending at 1586.48 cm-1 and 1392.68 cm-1, respectively, representative of primary amines. A sharp peak at 1054.79 cm-1 may be due to C-O stretching vibration for alcohols or phenols or may be due to C-N stretching vibration for amines.
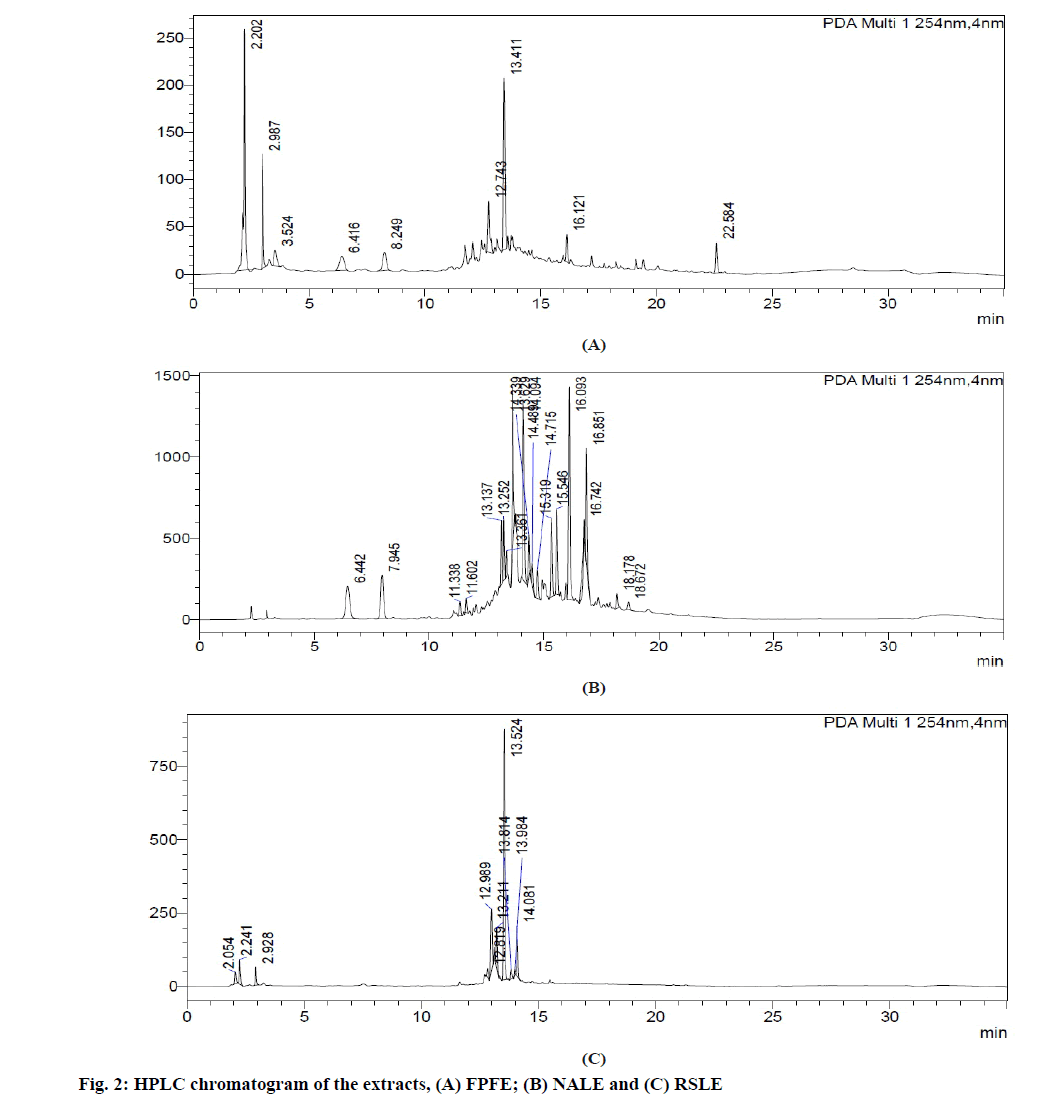
HPLC analysis of FPFE (fig. 2A) showed the presence of 9 compounds three of which correlated to gallic acid (Retention Time (Rt): 6.416 min), 1,3-O-caffeoylquinic acid (Rt:12.743 min) and epicatechin (Rt: 22.584 min) were found similar to that reported in earlier studies [45,46].
HPLC chromatogram of NALE (fig. 2B) showed the presence of 19 compounds. Four of these compounds correlated to gallic acid (Rt: 6.442 min), chlorogenic acid (Rt: 7.945 min) and iridoid glycosides (Rt: 15.319 and 18.67 min) as previously reported [47,48].
HPLC analysis of RSLE (fig. 2C) showed the presence of 10 compounds two of which is correlated to catechin (R : 12.989 min) and caffeic acid (R : 13.984 min) as in previous studies [5,49] t . Marker compounds were not estimated in the extracts, which is the limitation of the study.
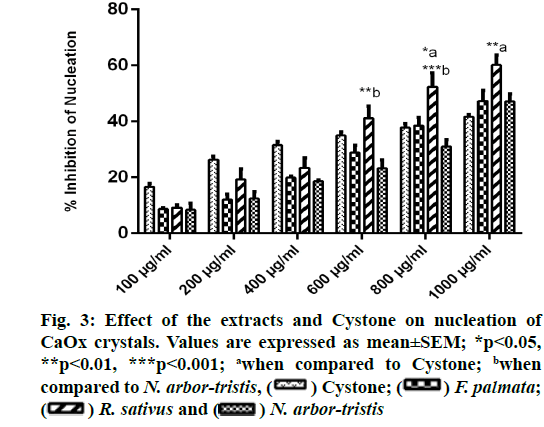
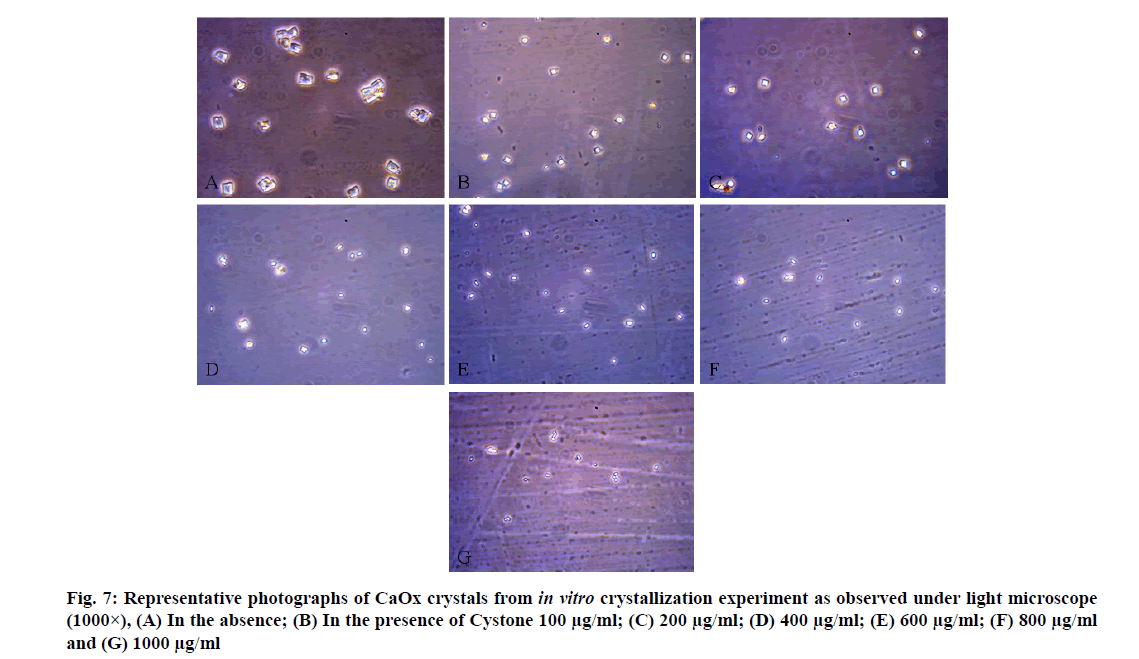
A concentration dependent rise in the reduction of CaOx crystallization was witnessed with all the three extracts and Cystone. RSLE showed significantly better outcomes in inhibiting nucleation of CaOx crystals as compared to Cystone at higher concentrations i.e. 800 µg/ml (p<0.05) and 1000 µg/ml (p<0.01) followed by FPFE and NALE. Percent inhibition of nucleation at highest concentration (1000 µg/ml) was recorded to be 60.14 %±3.57 % for RSLE, 47.33 %±3.25 % for FPFE, 47.12 %±2.74 % for NALE and 41.67 %±0.72 % for Cystone (fig. 3).
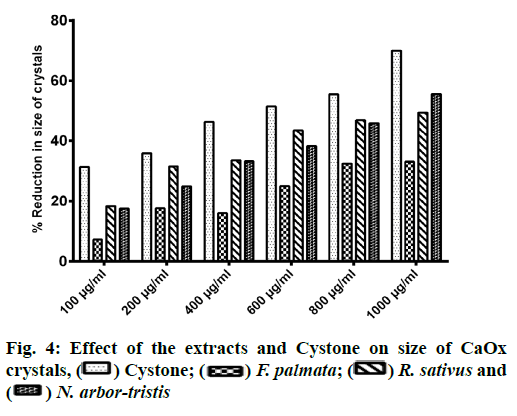
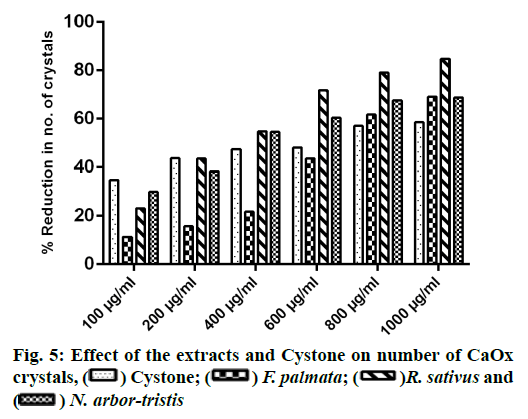
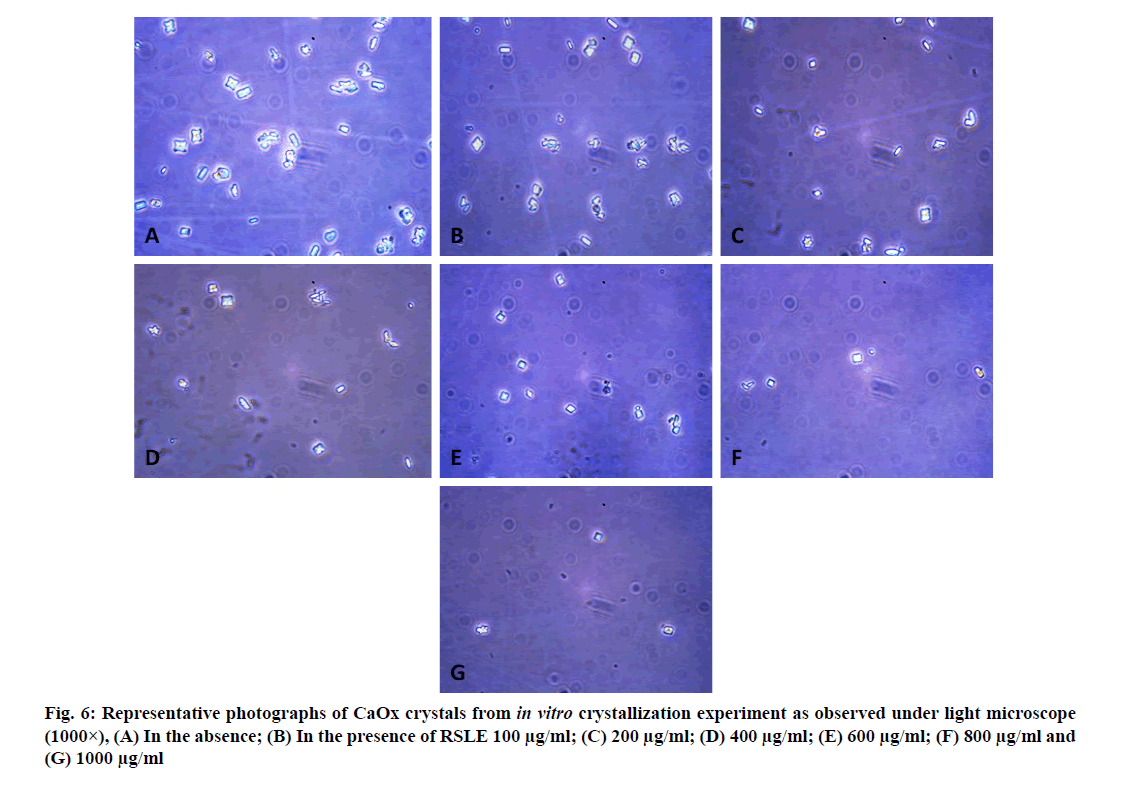
Microscopic evaluation of CaOx crystals revealed the beneficial outcomes of NALE, RSLE and FPFE in reducing the crystal abundance and size. When compared to the standard drug Cystone, the extracts were more effective in reducing the number of crystals, whereas Cystone produced a more pronounced effect in reducing the size of CaOx crystals (70.1 %). Of the three extracts, NALE (1000 µg/ml) produced highest percent reduction of the size of crystals (55.58 %), followed by RSLE (49.49 %) and FPFE (33.13 %) (fig. 4). Whereas, RSLE (1000 µg/ml) produced highest percent reduction of the number of crystals (84.75 %), followed by FPFE (69.14 %), NALE (68.83 %) and Cystone (58.52 %) (fig. 5).
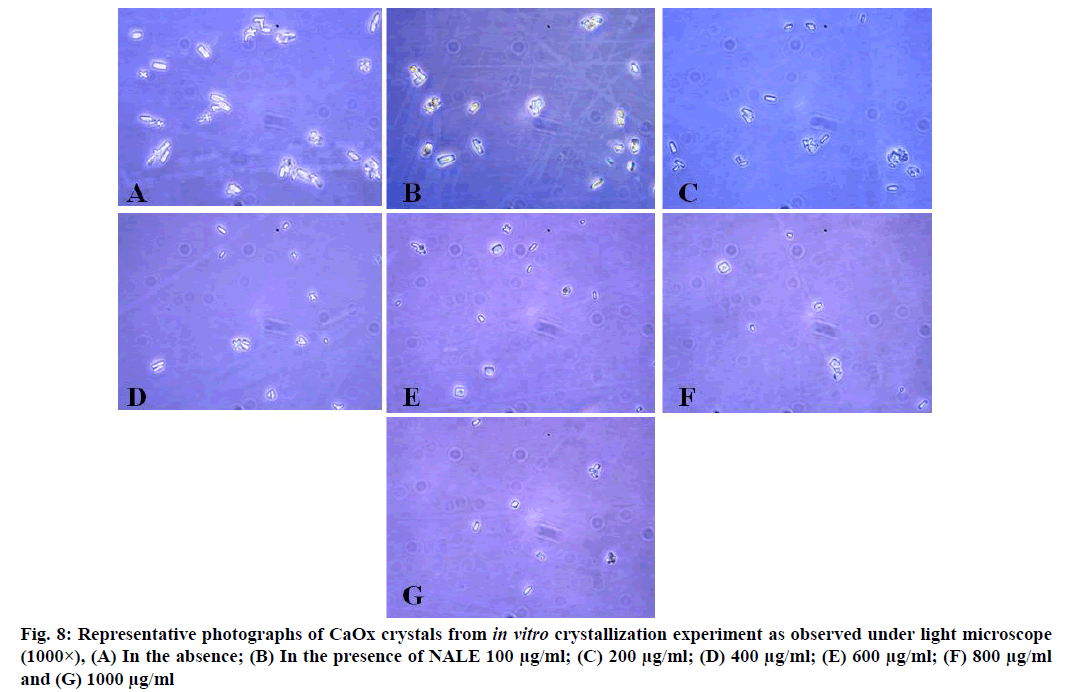
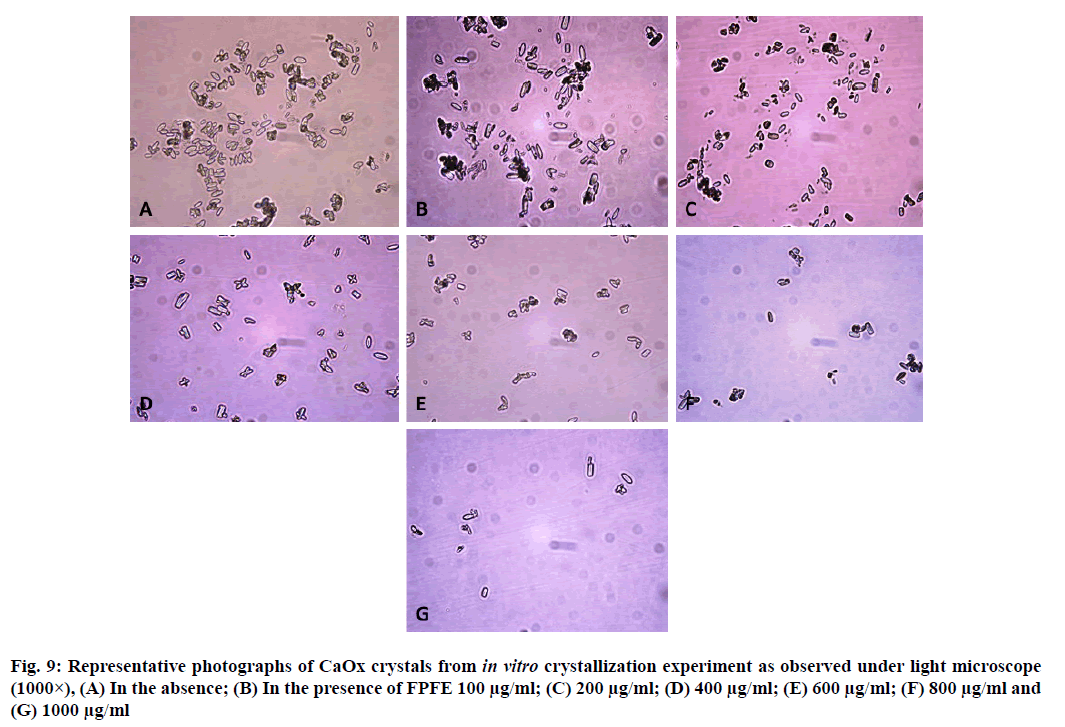
CaOx crystals in control group majorly exhibited monoclinic or rectangular habit characteristic of Calcium Oxalate Monohydrate (COM) crystals. Calcium Oxalate Dihydrate (COD) crystals that were present in the control group were few in number and that too with sharp edges. A morphological transformation of crystals from COM to tetragonal bipyramidal COD crystals with smooth surface and edges was witnessed in the presence of the extracts and Cystone. This effect was most prominent with RSLE (fig. 6) that produced favorable morphological change in majority of crystals at lowest concentration itself similar to that of Cystone (fig. 7). NALE promoted COD crystal formation at 600 µg/ml concentration and above (fig. 8). This effect on CaOx crystal morphology was less apparent with FPFE (fig. 9).
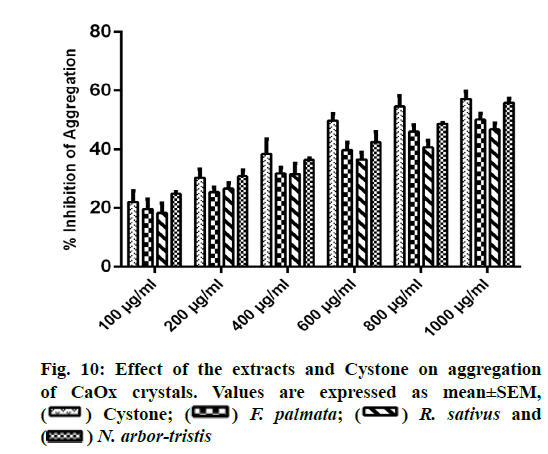
A concentration dependent increase in reducing CaOx aggregation was witnessed with all the three extracts and Cystone. NALE showed highest percent inhibition of aggregation viz. 55.83 %±1.56% at 1000 µg/ml comparable to that of Cystone (57.15 %±2.53 %). Percent inhibition of aggregation for FPFE and RSLE was recorded as 50.17 %±2.059 % and 46.85 %±2.12 %, respectively (fig. 10).
Concentration dependent inhibition of CaOx crystal growth was observed for all the extracts and Cystone. Percent inhibition of growth recorded for NALE was 51.79 %±4.05 %, for RSLE was 44.12 %±3.9 %, for FPFE was 39.35 %±4.7 % and for Cystone was 57.44 %±3.74%. Growth inhibitory efficacy of FPFE at highest concentration was found to be significantly (p<0.05) less than that of Cystone (fig. 11).
CaOx urolithiasis was addressed in the present study as it is the most challenging type of urolithiasis due to its majorly idiopathic nature [12] and complex etiology [13]. It also presents the most prevalent and recurrent class among all the urinary stone diseases [50].
Nucleation, growth and aggregation are key events among the myriad of steps involved in stone formation.
Hence, any alteration in the course of these events brought about by synthetic or natural substances can promote or inhibit calculi formation [51]. Nucleation, growth and aggregation assays that were used in the present study for evaluating the antiurolithiatic efficacy of the plant extracts are principally simulation of the crucial predisposing factors for CaOx stone formation inside the body. OD of the turbid solutions produced as a result of the formation of CaOx crystals on combining CaCl2 and Na2C2O4 solutions was measured spectrophotometrically as OD is directly proportional to turbidity (τ). This inference has been made from the expression τ=2.303 (OD/l) that was first devised by Melik and Fogler in the year 1983. In the expression ‘l’ stands for the path length [52].
Nucleation is a preliminary event that marks the process of spontaneous crystallization in a supersaturated solution. It also serves as a prerequisite to the crystallization and stone forming events that follow [51]. Henceforth, inhibition of nucleation can play a significant role in inhibiting stone formation. Present study showed beneficial outcomes of RSLE, NALE and FPFE in inhibiting nucleation of CaOx crystals which was even better than that of Cystone. This was also supported by the microscopic studies of the metastable solutions of CaOx crystals that showed fewer crystals in the presence of these extracts. This clearly reveals the ability of these extracts to form complex with calcium and oxalate ions which would have served as a possible mechanism to reduce relative supersaturation with respect to CaOx and thus inhibit CaOx crystallization. Similar mechanism has been reported for anticrystallization activity of Sarghassum wightti by Sujatha et al. [53]. Moreover, phytochemical investigation of the three extracts revealed the presence of tannins which are known to aid in calcium complexation and thus inhibit CaOx crystallization [54]. Since the FT-IR analysis of RSLE, FPFE and NALE showed the presence of O-H, C-N and -NHCO groups that are anionic in nature, it can be alleged that calcium complexation by these extracts would have been the more effective mechanism involved in reducing CaOx supersaturation [55].
Crystalgrowthis suggestive ofincreasein thedimensions of the crystals as a result of the deposition of atoms and molecules over the existing crystal lattice [56]. Size of the crystals is a crucial determinant of stone formation as large crystals pose a risk of occlusion and retention while smaller crystals spontaneously excrete out in urine [57]. In the present study RSLE, NALE and FPFE showed promising potential in CaOx crystal growth inhibition. This growth inhibitory effect of RSLE, NALE and FPFE was also evident from the smaller crystals produced in the presence of these extracts as conferred from the microscopic investigation. The growth inhibitory effect of the extracts may have resulted from adsorption of the phytoconstituents over the crystal surface that may have hindered the addition of cations and anions to the crystal lattice thus interfering with the growth of the crystals [58,59].
Crystal aggregation is the key determinant of stone formation process, as it accounts for crystal retention. Aggregation of crystals suggests clustering of numerous crystals to acquire enormous size. Crystal aggregates are a common finding in urolithiatic urine and in CaOx stone matrix [56]. In the present study RSLE, NALE and FPFE showed promising potential in inhibiting CaOx crystal aggregation. The antiaggregatory effect of the extracts would have been the consequence of the adsorption of the various phytoconstituents of the extracts over the CaOx crystal surface. Thereby, raising the zeta potential of the crystals rendering them more electronegative, thus hindering crystal- crystal interaction [60] by overcoming Van der Waals attraction force that hold the crystals together into aggregates [61]. This seems to be fairly possible as presence of numerous anionic moieties was confirmed through FT-IR analysis of the extracts that can impart negative charge to the crystals. Phytochemical analysis also revealed the presence of saponins and flavonoids in RSLE, NALE and FPFE. Flavonoids and saponins are known to induce disintegration and dissolution of CaOx crystals [62].
Polymorphic forms of CaOx crystals have a remarkable impact in the course of disease progression of urolithiasis. As compared to the COM crystals, COD crystals are less injurious to the renal epithelial cells. This is due to their reduced adhesive ability that deters their attachment to the renal epithelium as well as hinders their agglomeration [30]. Therefore, transformation of COM crystals to COD crystals that was witnessed in the presence of RSLE and NALE shows their immense potential as possible candidate for drug development for urolithiasis. These observations are in agreement with those reported for Herniaria hirsuta [57] and Holarrhena antidysenterica [30].
The IR spectra of FPFE, NALE and RSLE showed the presence of functional groups which are characteristic of phenolic compounds, carboxylic acids, amines [63], flavonoids and amino acids [64]. Moreover, peaks in FT-IR spectra of NALE indicated the presence of arbortristoside B, an iridoid glycoside [44].
HPLC analysis of FPFE showed the presence of gallic acid, 1,3-O-caffeoylquinic acid and epicatechin, that of NALE showed the presence of gallic acid, chlorogenic acid and iridoid glycosides, and HPLC chromatogram of RSLE showed the presence of catechin and caffeic acid. These polyphenolic compounds present in the extracts are of added advantage. They possess antioxidant activity which by inhibiting oxidation mediated renal tissue damage prevent crystal-cell interaction of CaOx crystals with the renal tissue and thus prevent further disease progression [3]. Chlorogenic acid, caffeic acid and gallic acid present in NALE, RSLE and FPFE, respectively have been reported to be strong iron chelators and hence possess strong ability to inhibit free radical generation and lipid peroxidation [65]. Moreover, chlorogenic acid [66], catechin [67] and caffeic acid found to be present in NALE and RSLE, respectively also possess anti-inflammatory activity [68]. Anti-inflammatory activity of these polyphenolic compounds may have the significance in providing symptomatic relief in urolithiasis [3]. Caffeic acid, chlorogenic acid [69] and catechin have also been reported to possess Angiotensin Converting Enzyme (ACE) inhibitory activity [70] and therefore may prove to be efficacious in ameliorating renal stone disease by inhibiting inflammation of renal tissue and CaOx crystal deposition [3]. Catechin has also been reported to possess antiurolithiatic activity against CaOx crystallization in in vitro and in vivo models of urolithiasis [19].
In vitro studies provide an optimum insight into the potential activity related outcomes of the extracts or compounds under investigation and also provide a platform for preliminary investigations that help in devising future studies. Present study demonstrated promising antiurolithiatic potential of the hydroethanolic extract of the fruits of F. palmata, leaves of N. arbor- tristis and R. sativus in in vitro setting of which N. arbor- tristis and R. sativus produced more pronounced effects in modulating each step of CaOx crystallization. Taking into account the effects of the lowest concentration of the three extracts, N. arbor-tristis possessed maximum anti-aggregatory and growth inhibitory activity against CaOx crystallization at the lowest tested concentration (100 µg/ml). This may have the outcome of the higher phenolic content of the NALE (3.504±0.137 mg GAE/g extract) as compared to RSLE and FPFE. Although, the anticrystallization activity of FPFE tested in in vitro settings in the present study was comparatively less as compared to the other tested extracts, nevertheless, F. palmata has been reported to be a plant of high use value with analgesic activity which may prove to be an added advantage in combating urinary stone disease in vivo [71].
Findings of the present study demonstrated the efficacy of R. sativus leaves, F. palmata fruits and N. arbor-tristis leaves in favorably modulating nucleation, growth and aggregation phases of CaOx crystallization events of stone formation in in vitro settings. Prominent advocation of COD crystallization and suppression of COM crystal formation was witnessed in the presence of R. sativus leaves and N. arbor-tristis leaves. All these effects can be attributed to the saponins, tannins, flavonoids and polyphenolic principles of the tested extracts, and to the ability of the extracts to raise the zeta potential of the CaOx crystals to inhibit attachment of ionic entities to growing crystal lattice and crystal-crystal interaction. Further exploration of the antiurolithiatic potential of these plant extracts in preclinical and clinical settings and characterization of the active constituents may lead to the development of new plant based molecules or products for the treatment and prevention of urolithiasis. R. sativus leaves are widely grown and consumed worldwide and hence can be a solution to the enigmatic recurrence of the urinary stone disease in the afflicted individuals in form of a common consumable house hold commodity.
Acknowledgements:
First author is thankful to the Department of Science and Technology (Innovation in Science Pursuit for Inspired Research (INSPIRE) program, [Grant number DST/ INSPIRE Fellowship/2014/IF140276]), Government of India, for providing financial assistance to carry out the related research work. The authors are also thankful to Mr. B. K. Singh, Associate Professor, Department of Pharmaceutical Sciences, Kumaun University, Nainital and Dr. L.S. Rautela, Laboratory Technician, Department of Pharmaceutical Sciences, Kumaun University, Nainital for their undeniable support and for providing amenities to carry out the research work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Makbul SA, Wadud A, Jahan N, Sofi G, Khan MI. Scientific appraisal of urolithiasis and its remedial measures in Unani medicine. J Herb Med 2017;8:1-7.
- Shoag J, Tasian GE, Goldfarb DS, Eisner BH. The new epidemiology of nephrolithiasis. Adv Chronic Kidney Dis 2015;22(4):273-8.
[Crossref] [Google Scholar] [PubMed]
- Ahmed S, Hasan MM, Khan H, Mahmood ZA, Patel S. The mechanistic insight of polyphenols in calcium oxalate urolithiasis mitigation. Biomed Pharmacother 2018;106:1292-9.
[Crossref] [Google Scholar] [PubMed]
- Aggarwal KP, Tandon S, Naik PK, Singh SK, Tandon C. Novel antilithiatic cationic proteins from human calcium oxalate renal stone matrix identified by MALDI-TOF-MS endowed with cytoprotective potential: An insight into the molecular mechanism of urolithiasis. Clin Chim Acta 2013;415:181-90.
[Crossref] [Google Scholar] [PubMed]
- Fakheri RJ, Goldfarb DS. Ambient temperature as a contributor to kidney stone formation: Implications of global warming. Kidney Int 2011;79(11):1178-85.
[Crossref] [Google Scholar] [PubMed]
- Alatab S, Pourmand G, El Howairis ME, Buchholz N, Najafi I, Pourmand MR, et al. National profiles of urinary calculi (a comparison between developing and developed worlds). Iran J Kidney Dis 2016;10(2):51-61.
[Google Scholar] [PubMed]
- López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol 2010;25(1):49-59.
[Crossref] [Google Scholar] [PubMed]
- Malhotra KK. Medical aspects of renal stones. J Indian Acad Clin Med 2008;9(4):282-6.
- Tiselius HG. A hypothesis of calcium stone formation: An interpretation of stone research during the past decades. Urol Res 2011;39(4):231-43.
[Crossref] [Google Scholar] [PubMed]
- Cloutier J, Villa L, Traxer O, Daudon M. Kidney stone analysis: “Give me your stone, I will tell you who you are!” World J Urol 2015 Feb;33(2):157-69.
[Crossref] [Google Scholar] [PubMed]
- Pearle MS. Prevention of nephrolithiasis. Curr Opin Nephrol Hypertens 2001;10(2):203-9.
[Google Scholar] [PubMed]
- Marangella M, Vitale C, Bagnis C, Petrarulo M, Tricerri A. Use of drugs for nephrolithiasis. Clin Cases Miner Bone Metab 2008;5(2):131-4.
[Google Scholar] [PubMed]
- Yasir F, Waqar MA. Effect of indigenous plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urol Res 2011;39(5):345-50.
[Crossref] [Google Scholar] [PubMed]
- Bartges JW, Callens AJ. Urolithiasis. Vet Clin North Am Small Anim Pract 2015;45(4):747-68.
[Crossref] [Google Scholar] [PubMed]
- Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 2015;14(2):111-29.
[Crossref] [Google Scholar] [PubMed]
- Peerapen P, Thongboonkerd V. Caffeine prevents kidney stone formation by translocation of apical surface annexin A1 crystal-binding protein into cytoplasm: In vitro evidence. Sci Rep 2016;6(1):1-3.
- Zhai W, Zheng J, Yao X, Peng B, Liu M, Huang J, et al. Catechin prevents the calcium oxalate monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complement Altern Med 2013;13(1):228-38.
[Crossref] [Google Scholar] [PubMed]
- Hong SH, Lee HJ, Sohn EJ, Ko HS, Shim BS, Ahn KS, et al. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol Rep 2013;65(4):970-9.
[Crossref] [Google Scholar] [PubMed]
- Ghodasara J, Pawar A, Deshmukh C, Kuchekar B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacol Rep 2010;2(6):388-92.
[Crossref] [Google Scholar] [PubMed]
- Zhu W, Xu YF, Feng Y, Peng B, Che JP, Liu M, et al. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis 2014;42(6):519-26.
[Crossref] [Google Scholar] [PubMed]
- Tewari D, Sah AN, Bawari S, Sharma H, Mangal AK. Pharmacognostical evaluation and HPTLC fingerprinting identification of Ficus palmata Forssk. (Bedu) from Western Himalaya. Curr Bioact Compd 2018;14(2):180-90.
- Alqasoumi SI, Basudan OA, Al-Rehaily AJ, Abdel-Kader MS. Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia. Saudi Pharm J 2014;22(5):460-71.
[Crossref] [Google Scholar] [PubMed]
- Sasmal D, Das S, Basu SP. Diuretic activity of Nyctanthes arbor-tristis Linn. Anc Sci Life 2007;27(2):19-23.
[Google Scholar] [PubMed]
- Vargas S R, Perez G RM, Perez G S, Zavala S MA, Perez G C. Antiurolithiatic activity of Raphanus sativus aqueous extract on rats. J Ethnopharmacol 1999;68(1):335-8.
[Crossref] [Google Scholar] [PubMed]
- Mute V, Awari D, Vawhal P, Kulkarni A, Bartakke U, Shetty R. Evaluation of diuretic activity of aqueous extract of Raphanus sativus. Eur J Biol Sci 2011;3:13-5.
- Beevi SS, Narasu ML, Gowda BB. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum Nutr 2010;65(1):8-17.
[Crossref] [Google Scholar] [PubMed]
- Saha RK, Acharya S, Shovon SS, Apu AS, Roy P. Biochemical investigation and biological evaluation of the methanolic extract of the leaves of Nyctanthes arbor-tristis in vitro. Asian Pac J Trop Biomed 2012;2(3):S1534-41.
- Saini R. Comparative study of three wild edible fruits of Uttrakhand for antioxidant, antiproliferative activities and polyphenolic composition. Int J Pharm Biosci 2012;3(4):158-67.
- Kothiyal SC, Saklani S. Isolation and identification of Ficus palmata leaves and their antimicrobial activities. J Sci Res 2017;9(2):193-200.
- Pant S, Samant SS, Arya SC. Diversity and indigenous household remedies of the inhabitants surrounding Mornaula reserve forest in West Himalaya. Indian J Tradit Knowl 2009;8(4), 606-10.
- Kakoti BB, Pradhan P, Borah S, Mahato K, Kumar M. Analgesic and anti-inflammatory activities of the methanolic stem bark extract of Nyctanthes arbor-tristis Linn. Biomed Res Int 2013;2013.
[Crossref] [Google Scholar] [PubMed]
- Aqil F, Ahmad I. Broad-spectrum antibacterial and antifungal properties of certain traditionally used Indian medicinal plants. World J Microbiol Biotechnol 2003;19(6):653-7.
- Khan A, Khan SR, Gilani AH. Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol Res 2012;40(6):671-81.
[Crossref] [Google Scholar] [PubMed]
- El Menyiy N, Al Waili N, Bakour M, Al-Waili H, Lyoussi B. Protective effect of propolis in proteinuria, crystaluria, nephrotoxicity and hepatotoxicity induced by ethylene glycol ingestion. Arch Med Res 2016;47(7):526-34.
[Crossref] [Google Scholar] [PubMed]
- Kokate CK, Parikh KN. Practical pharmacognosy. 18th ed. New Delhi: Vallabh Prakashan; 2017.
- Khandelwal K. Practical pharmacognosy techniques and experiments. 2nd ed. Pune: Nirali Prakashan; 2000.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 1999;299:152-78.
- Al-Owaisi M, Al-Hadiwi N, Khan SA. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringaperegrina (Forssk.) Fiori leaves. Asian Pac J Trop Biomed 2014;4(12):964-70.
- Manissorn J, Fong-Ngern K, Peerapen P, Thongboonkerd V. Systematic evaluation for effects of urine pH on calcium oxalate crystallization, crystal-cell adhesion and internalization into renal tubular cells. Sci Rep 2017;7(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. And Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 2012;144(1):160-70.
[Crossref] [Google Scholar] [PubMed]
- Mittal A, Tandon S, Singla SK, Tandon C. In vitro studies reveal antiurolithic effect of Terminalia arjuna using quantitative morphological information from computerized microscopy. Int Braz J Urol 2015;41:935-44.
[Google Scholar] [PubMed]
- Devkar RA, Chaudhary S, Adepu S, Xavier SK, Chandrashekar KS, Setty MM. Evaluation of antiurolithiatic and antioxidant potential of Lepidagathis prostrata: A Pashanbhed plant. Pharm Biol 2016;54(7):1237-45.
[Crossref] [Google Scholar] [PubMed]
- Chaudhary A, Singla SK, Tandon C. In vitro evaluation of Terminalia arjuna on calcium phosphate and calcium oxalate crystallization. Indian J Pharm Sci 2010;72(3):340-5.
[Crossref] [Google Scholar] [PubMed]
- Purushothaman KK, Venkatanarasimhan M, Sarada A. Arbortristoside A and B, two iridoid glucosides from Nyctanthes arbor-tristis. Phytochemistry 1985;24(4):773-6.
- Faleh E, Oliveira AP, Valentão P, Ferchichi A, Silva BM, Andrade PB. Influence of Tunisian Ficus carica fruit variability in phenolic profiles and in vitro radical scavenging potential. Rev Bras Farmacogn 2012;22(6):1282-9.
- Sumi SA, Siraj M, Hossain A, Mia M, Afrin S, Rahman M. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid Based Complement Alternat Med 2016;2016.
[Crossref] [Google Scholar] [PubMed]
- Meghashri S, Gopal S. Biochemical characterization of radical scavenging polyphenols from Nyctanthes arbor-tristis. J Pharm Bioallied Sci 2012;4(4):341-4.
[Google Scholar] [PubMed]
- Agrawal J, Shanker K, Chanda D, Pal A. Nyctanthes arbor-tristis positively affects immunopathology of malaria-infected mice prolonging its survival. Parasitol Res 2013;112(7):2601-9.
[Crossref] [Google Scholar] [PubMed]
- Eugenio MH, Pereira RG, Abreu WC, Pereira MC. Phenolic compounds and antioxidant activity of tuberous root leaves. Int J Food Prop 2017;20(12):2966-73.
- Alelign T, Petros B. Kidney stone disease: An update on current concepts. Adv Urol 2018;2018.
[Crossref] [Google Scholar] [PubMed]
- Basavaraj DR, Biyani CS, Browning AJ, Cartledge JJ. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Updat Ser 2007;5(3):126-36.
- Troy DB. Remington: The science and practice of pharmacy. 21st ed. Philadelphia, USA: Lippincott Williams & Wilkins; 2005.
- Sujatha D, Singh K, Vohra M, Kumar KV, Sunitha S. Antilithiatic activity of phlorotannin rich extract of Sarghassum wightii on calcium oxalate urolithiais–in vitro and in vivoevaluation. Int Braz J Urol 2015;41:511-20.
[Crossref] [Google Scholar] [PubMed]
- Doddola S, Pasupulati H, Koganti B, Prasad KV. Evaluation of Sesbania grandiflora for antiurolithiatic and antioxidant properties. J Nat Med 2008;62(3):300-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang CY, Wu WH, Wang J, Lan MB. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar Drugs 2012;10(1):119-30.
[Crossref] [Google Scholar] [PubMed]
- Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int 2013;2013.
[Crossref] [Google Scholar] [PubMed]
- Atmani F, Khan SR. Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in vitro. BJU Int 2000;85(6):621-5.
[Crossref] [Google Scholar] [PubMed]
- Farmanesh S, Chung J, Sosa RD, Kwak JH, Karande P, Rimer JD. Natural promoters of calcium oxalate monohydrate crystallization. J Am Chem Soc 2014;136(36):12648-57.
[Crossref] [Google Scholar] [PubMed]
- Singh VK, Rai PK. Kidney stone analysis techniques and the role of major and trace elements on their pathogenesis: A review. Biophys Rev 2014;6(3):291-310.
[Crossref] [Google Scholar] [PubMed]
- Mittal A, Tandon S, Singla SK, Tandon C. Cytoprotective and anti-apoptotic role of Terminalia arjuna on oxalate injured renal epithelial cells. Cytotechnology 2017;69(2):349-58.
[Crossref] [Google Scholar] [PubMed]
- Baumann JM, Affolter B, Caprez U, Henze U. Calcium oxalate aggregation in whole urine, new aspects of calcium stone formation and metaphylaxis. Eur Urol 2003;43(4):421-5.
[Crossref] [Google Scholar] [PubMed]
- Sikarwar I, Dey YN, Wanjari MM, Sharma A, Gaidhani SN, Jadhav AD. Chenopodium album Linn. leaves prevent ethylene glycol-induced urolithiasis in rats. J Ethnopharmacol 2017;195:275-82.
[Crossref] [Google Scholar] [PubMed]
- Maobe MA, Nyarango RM, Box PO. Fourier transformer infra-red spectrophotometer analysis of Urtica dioica medicinal herb used for the treatment of diabetes, malaria and pneumonia in Kisii region, Southwest Kenya. World Appl Sci J 2013;21(8):1128-35.
- Oliveira RN, Mancini MC, Oliveira FC, Passos TM, Quilty B, Thiré RM, et al. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio de Janeiro) 2016;21:767-79.
- Andjelkovi? M, Van Camp J, De Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, et al. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem 2006;98(1):23-31.
- Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res 2014;63(1):81-90.
[Crossref] [Google Scholar] [PubMed]
- El-Aziz A, Tarek A, Mohamed RH, Pasha HF, Abdel-Aziz HR. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med 2012;12(4):233-40.
[Crossref] [Google Scholar] [PubMed]
- Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab 2009;6(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Bhullar KS, Lassalle-Claux G, Touaibia M, Rupasinghe HV. Antihypertensive effect of caffeic acid and its analogs through dual renin–angiotensin–aldosterone system inhibition. Eur J Pharmacol 2014;730:125-32.
[Crossref] [Google Scholar] [PubMed]
- He J. Bioactivity-guided fractionation of pine needle reveals catechin as an anti-hypertension agent via inhibiting angiotensin-converting enzyme. Sci Rep 2017;7(1):1-9.
- Tiwari D, Sah AN, Bawari S, Bussmann RW. Ethnobotanical investigations on plants used in folk medicine by native people of Kumaun Himalayan Region of India. Ethnobot Res Appl 2020;20:1-35.