- *Corresponding Author:
- Patthraporn Siripipatthana
Department of Chemistry,
Protein and Enzyme Technology Research Unit,
Faculty of Science,
Mahasarakham University,
Kantharawichai,
Maha Sarakham 44150,
Thailand
E-mail: patthraporn.s@msu.ac.th
| Date of Received | 18 August 2021 |
| Date of Revision | 24 October 2021 |
| Date of Acceptance | 22 July 2022 |
| Indian J Pharm Sci 2022;84(4):929-937 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to evaluate and correlate phytochemical content, antioxidant activity and alpha-amylase inhibitory activity in aqueous acetone extract and fractions derived from it (ethyl acetate fraction, watersoluble fraction, aqueous methanol fraction and aqueous acetone fraction) which are obtained from Ampelocissus martini Planch. root. Ethyl acetate fraction and aqueous acetone fraction had the highest total phenolic content and total proanthocyanidin content, respectively. 2,2-diphenyl-1-picrylhydrazyl, 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid), cupric reducing antioxidant capacity and ferric reducing antioxidant power assays showed that aqueous acetone fraction had higher antioxidant activity compared to other samples and standards. Acarbose and all samples except aqueous methanol fraction inhibited alpha-amylase, a key enzyme linked to type 2 diabetes, in a dose-dependent manner and aqueous acetone fraction showed the strongest inhibition (half-maximal inhibitory concentration=8.77±0.28 μg/ml). Aqueous acetone fraction was a mixed noncompetitive inhibitor of the enzyme. Correlation analysis showed strong positive correlations among proanthocyanidin content, antioxidant activity and alphaamylase inhibitory activity. These results suggest that aqueous acetone fraction, proanthocyanidins-rich fraction from Ampelocissus martini root, may be used for effective diabetes management because of its high potential antioxidant activity and alpha-amylase inhibitory activity.
Keywords
Ampelocissus martini, proanthocyanidins, alpha-amylase inhibitory activity, antioxidant activity, antidiabetic activity
Diabetes mellitus is a group of disorders generally characterized by hyperglycemia resulting from a deficiency in insulin secretion and/or insulin action[1]. The disease is rapidly growing worldwide with an increasing morbidity and mortality[2]. The most common type of diabetes in adults is type 2 diabetes[3]. Hyperglycemia can induce the excessive generation of free radicals resulting in oxidative stress, which further increases the progression of many diseases including diabetes[4,5]. The chronic hyperglycemia of diabetes causes damage and failure of many organs including the eyes, heart and kidneys[1].
Alpha (α)-amylase (EC 3.2.1.1) or 4-α-D-glucan glucanohydrolase is one of two key enzymes linked to type 2 diabetes and is found in saliva and small intestine of the human body. It hydrolyzes complex polysaccharides to oligosaccharides which are then hydrolyzed by intestinal α-glucosidase to liberate glucose before entering the bloodstream[6]. Inhibition of α-amylase by inhibitors will reduce postprandial blood glucose levels. Acarbose, inhibitor of α-amylase has been used for the management of hyperglycemia and type 2 diabetes, but with undesirable side effects including diarrhea and flatulence[7]. Finding natural and safer enzyme inhibitors with strong antioxidant activity has been proposed for the treatment of diabetes since it can control hyperglycemia and diabetes complications caused from oxidative stress[8-10].
Phenolic compounds are widely distributed in the plant kingdom and classified into several groups based on the number of phenol rings and structural elements that bind these rings to one another[11,12]. Their diverse structures greatly influence their solubility in water and organic solvents[13]. Phenolic compounds including proanthocyanidins which contain antioxidant activity and α-amylase inhibitory activity have been considered as a potential antidiabetic agents in management of type 2 diabetes[3,6,14,15]. Ampelocissus martini (A. martini) Planch. is a wild grape commonly found in Thailand. Leaves, roots and bark of the plant have been used as ingredients in Thai traditional medicine to provide relief of symptoms. Many parts of the plant such as the fruits, vine, rhizome, leaves contain high concentrations of phenolic compounds with antioxidant activity[16,17]. Phenolic compounds such as gallic acid, caffeic acid, resveratrol, catechin, epicatechin, rutin and quercetin are present in seeds[18]. Antibacterial activity has also been found in fruits of this plant[19]. However, limited information is available on the α-amylase inhibitory activity of extracts from root of A. martini as well as the relationship among content of phenolics and proanthocyanidins, antioxidant and antidiabetic activities. In this study, phenolic and proanthocyanidin content, in vitro antioxidant activity and α-amylase inhibitory activity from A. martini root extract and its fractions were determined and correlated.
Materials and Methods
Chemicals and reagents:
All chemicals and reagents were of analytical grade. Hydrochloric acid (HCl), petroleum ether, ethyl acetate, methanol, ethanol and acetic acid were purchased from QRec (Auckland, New Zealand). Gallic acid, vanillin, (+)-catechin, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), 2,2' Azino Bis (3-Ethylbenzthiazoline-6-Sulphonic Acid) diammonium salt (ABTS), 2,9-dimethyl-1,10- phenanthroline (Neocuproine), sephadex-LH 20, potato starch, α-amylase from porcine pancreas type VI-B, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) and 6-hydroxy- 2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox) were obtained from Sigma-Aldrich (Missouri, United States of America (USA)). Sodium carbonate (Na2CO3), Potassium persulfate (K2S2O8), Ferric chloride (FeCl3), Copper (II) chloride dihydrate (CuCl2.2H2O), Iron (II) sulfate heptahydrate (FeSO4.7H2O) and Sodium acetate trihydrate (CH3COONa.3H2O) were obtained from Ajax Finechem (Auckland, New Zealand). Ascorbic acid, Sodium chloride (NaCl), Dimethyl Sulfoxide (DMSO), 3,5-dinitrosalicylic acid and Folin-Ciocalteu’s reagent were obtained from Carlo Erba (Milan, Italy). Quercetin, Butylated Hydroxytoluene (BHT) and acarbose were purchased from Acros Organic (New Jersey, USA).
Plant material and sample preparation:
A. martini was collected from the wild in Roi-Et Province, Northeastern Thailand in December 2019. A dry specimen of the plant number Siripipatthana 1 was deposited at the Khon Kaen University (KKU) herbarium, Department of Biology, Faculty of Science, Khon Kaen University, Khon Kaen, Thailand. Roots of the plant were used in the current study. The roots were washed and dried in shade. An electronic grinder were used to grind the dried roots into a fine powder which was then stored in an air-tight container at 25° in darkness.
Different solvent extractions and gel chromatographic fractionation:
Method of Chen et al.[20] with some modifications was used. Dried root powder (20 g) was extracted for 1.5 h with 700 ml of 70 % aqueous acetone at 25° using a magnetic stirrer (Clifton® Ceraplate; Nickel-Electro, United Kingdom (UK)). Centrifugation at 2200 g for 20 min (Rotanta 46R; Andreas Hettich GmbH and Co. KG, Germany) yielded a supernatant and pellet. The pellet was re-extracted 2 times using the same procedure and the three resulting supernatants were pooled and then filtered using Whatman no.1 paper (GE Healthcare, UK). Acetone was removed using a rotary vacuum evaporator (Hei-VAP g3; Heldolph instrument Gmbh and Co. KG, Germany) at 40° to obtain an aqueous extract. The aqueous extract was divided into 2 parts. The first part was freeze-dried to yield crude extract (Aqueous Acetone Extract (AAE)). The second one was extracted with hexane (300 ml) and then with petroleum ether (300 ml) to remove lipophilic compounds prior to fractionation using the modified methods[21,22]. The remaining aqueous extract was extracted with ethyl acetate (3×300 ml). Two fractions were obtained; Ethyl Acetate Fraction (EF) and the Water-Soluble Fraction (WF). The EF was dried using a rotary vacuum evaporator at 40° while the WF was freeze-dried. Next, 7 ml of 20 mg/ ml WF was prepared in 50 % aqueous methanol and chromatographed on a Sephadex LH-20 column (2×50 cm). Elution was performed at a flow rate of 0.5 ml/min with 50 % aqueous methanol and then with 70 % aqueous acetone and two fractions retained; Aqueous Methanol Fraction (AMF) and Aqueous Acetone Fraction (AAF). Finally, the AMF and AAF were dried using vacuum evaporation and freeze-drying. For freeze-drying, each fraction was kept at -40° in a deep freezer for 24 h, then was lyophilized under vacuum (0.4 mbar) using a freeze-dryer (Alpha 3-4 LSCbasic; Martin Christ GmbH, Germany). 50 % DMSO was prepared in distilled water. AAE and all fractions were separately dissolved in 50 % DMSO to yield stock solution (20 mg/ml).
Analysis of Total Phenolic Content (TPC):
TPC was evaluated according to the method of Farhadi et al.[23] with slight modification. Briefly, the sample was diluted to appropriate concentration in 50 % DMSO. An aliquot of 0.4 ml of sample was mixed with 2 ml of 10 % Folin-Ciocalteu’s reagent and incubated for 5 min at 25° prior to adding 1.6 ml of 7.5 % (w/v) Na2CO3. The mixture was allowed to stand for 30 min at 25°, followed by measurement of absorbance at 765 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA). A blank was prepared as above but 50 % DMSO was used instead of sample. The TPC of samples was reported as milligram of Gallic Acid Equivalent per gram of Dry Weight (mg GAE/g DW).
Analysis of Total Proanthocyanidin Content (TPAC):
TPAC assay was based on a procedure reported by Li et al.[24]. Each sample was diluted to appropriate concentration in distilled water. The sample solution (0.25 ml) was mixed with 1.5 ml of 4 % (w/v) vanillin in ethanol (freshly prepared) in a test tube, followed by addition of 0.75 ml of 37 % (w/w) HCl. The tube was capped and left for 15 min at 25° prior to the measurement of absorbance at 500 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA). A blank was prepared as above but 50 % DMSO was used instead of sample. The TPAC of each sample was reported as milligram of Catechin Equivalent per gram of Dry Weight (mg CE/g DW).
DPPH assay:
The ability of samples to scavenge DPPH radicals (DPPH•) was evaluated based on a modified method[23]. Briefly, 0.1 mM of DPPH• was freshly prepared by dissolving DPPH in methanol and 1 ml of this solution was mixed with 0.5 ml of various concentrations of sample (5-30 µg/ml of AAE, 5-50 µg/ml of EF, 5-25 µg/ml of WF, 10-60 µg/ml of AMF, 2.5-12.5 µg/ml of AAF, 12.5-100 µg/ml of BHT, 1-10 µg/ml of ascorbic acid and 1-16 µg/ml of Trolox) or 50 % DMSO (negative control). The mixture was allowed to stand for 30 min at 25° in the dark and then the absorbance was measured at 517 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA) using 50 % DMSO as the blank. Percentage (%) of DPPH inhibition was calculated based on equation (1).
DPPH inhibition (%)=[(A0–AS)/A0]×100 (1)
Where A0=Absorbance of mixture in the absence of sample (negative control) and AS=Absorbance of mixture in the presence of sample.
A graph was constructed by plotting % inhibition against sample concentration. The antioxidant activity was expressed as the half maximal Inhibitory Concentration (IC50) value which is the concentration required to cause 50 % inhibition. BHT, ascorbic acid and Trolox were employed as positive controls.
ABTS assay:
The ABTS assay described by Re et al.[25] was used to measure ABTS radical cation (ABTS•+) scavenging activity of samples. To produce ABTS•+ solution, 7 mM of ABTS diammonium salt solution was mixed with 2.45 mM K2S2O8 at the volume ratio of 1:1, the mixture was left to stand in the dark at 25° for 16 h and then diluted with distilled water to absorbance at 0.700±0.020 at 734 nm. A volume of 0.5 ml of sample or 50 % DMSO (negative control) was mixed with 1 ml of the ABTS•+ solution. After 6 min of incubation at 25° in the dark, the absorbance of the reaction was measured at 734 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA), using 50 % DMSO as the blank. The percent inhibition was calculated similarly to DPPH inhibition and antioxidant activity was expressed as IC50 value. BHT, ascorbic acid and Trolox were also used as positive controls.
Cupric Reducing Antioxidant Capacity (CUPRAC) assay:
The method of Apak et al.[26] with some modifications was performed to evaluate CUPRAC value. Briefly, 0.5 ml of 10 mM CuCl2 solution was mixed with 0.5 ml of 7.5 mM Neocuproine in 96 % ethanol. The sample was diluted to appropriate concentration in 50 % DMSO and 0.55 ml of the diluted sample or 50 % DMSO (for blank) was added to the previous mixture solution. The reaction solution was allowed to stand for 30 min at 25°, followed by measurement of absorbance at 450 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA). The CUPRAC values of samples was calculated using a standard curve of Trolox and reported as milligram of Trolox Equivalent per gram of Dry Weight (mg TE/g DW).
Ferric Reducing Antioxidant Power (FRAP) assay:
The method of Zhang et al.[27] was used with slight modification. Firstly, FRAP reagent was prepared by mixing 10 mM TPTZ in 40 mM HCl, 20 mM FeCl3 and 300 mM sodium acetate buffer (pH 3.6) in the ratio of 10:10:100 (v/v/v). Then 3 ml of the reagent was mixed with 0.1 ml of sample or 50 % DMSO (for blank). After incubation for 15 min at 25°, the absorbance of the mixture at 593 nm was measured (Genesys 20 4001/4; ThermoFisher Scientific, USA). The FRAP value of samples was expressed as micromole Ferrous ion (Fe2+) equivalent/g Dry Weight (µmol Fe2+/g DW).
α-Amylase inhibition assay:
The methods of Wickramarantne et al.[28] and Olaokun et al.[29] were performed with minor modifications. AAE and its fractions at varying concentrations, 2 units/ ml of α-amylase, and 1 % (w/v) soluble potato starch were prepared in 20 mM sodium phosphate buffer (pH 6.9) containing 6.7 mM NaCl. A volume of 0.2 ml of the sample was mixed with 0.2 ml of α-amylase and then incubated for 10 min at 37°. Soluble potato starch (0.2 ml) was added and incubated for 5 min at 37°. To terminate the reaction, 0.2 ml of 3,5-dinitrosalicylic acid reagent was added to the mixture. The mixture was boiled for 10 min at 90° and then cooled to 25°. Distilled water (1.5 ml) was added to dilute the mixture prior to absorbance measurement at 540 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA). For the control, 0.2 ml of the sample solvent was used instead of sample. For the blank, the enzyme solution was replaced by the buffer. Acarbose was used as a positive control. The α-amylase inhibitory activity was calculated as percentage (%) inhibition, based on equation (2).
% Inhibition=[(Acontrol-Ablank)-(Asample-Ablank)/(Acontrol-Ablank)]×100 (2)
Where Acontrol=Absorbance of the control, Ablank=Absorbance of the blank and Asample=Absorbance of the sample.
The concentration of the sample resulting in a 50 % inhibition of α-amylase activity (IC50) was calculated from a graph plot of concentration against % inhibition.
Mode of α-amylase inhibition:
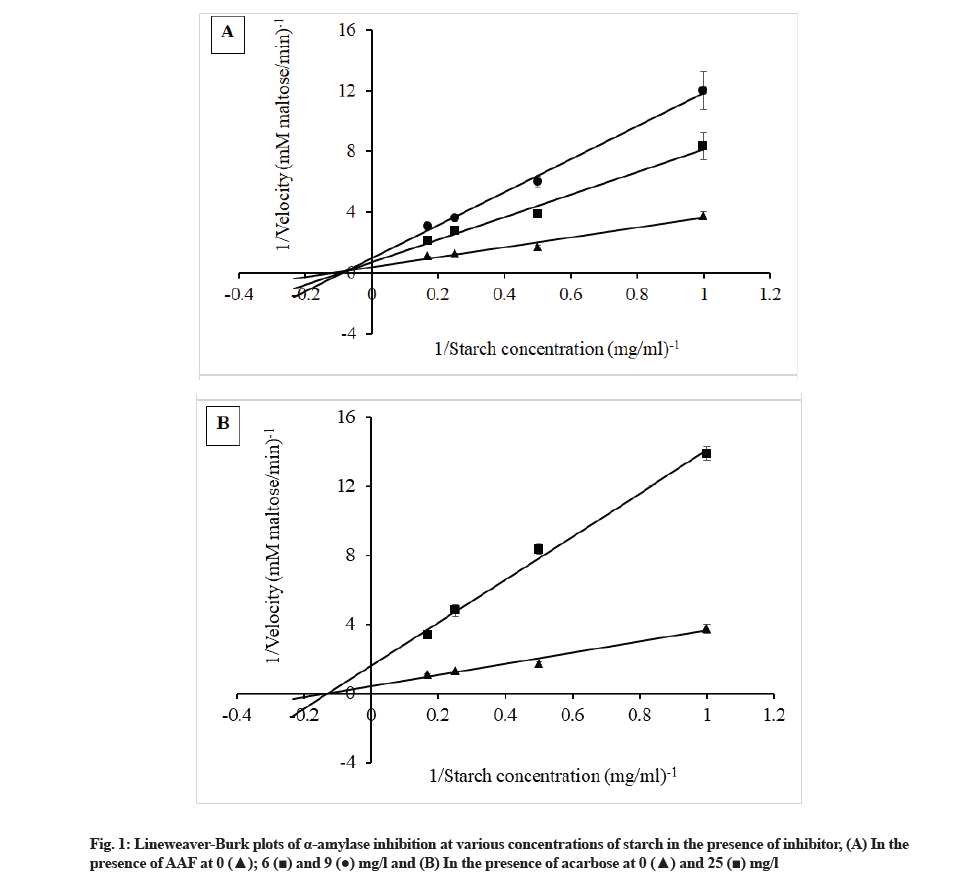
The method of Kazeem et al.[30] with some modifications was used to evaluate mode (type) of α-amylase inhibition. Two set of tubes were prepared. In the former set, 0.2 ml of AAF (6 mg/l and 9 mg/l) or standard acarbose (25 mg/l) was pre-incubated with 0.2 ml of α-amylase (2 units/ml) for 10 min at 37°. In the latter set, 0.2 ml of 20 mM sodium phosphate buffer (pH 6.9) containing 6.7 mM NaCl was pre-incubated with 0.2 ml of α-amylase. Then, 0.2 ml of soluble potato starch (1, 2, 4 and 6 mg/ml) was added to both sets and incubated for 5 min at 37°, followed by addition of 0.2 ml of 3,5-dinitrosalicylic acid reagent and boiling for 10 min at 90°. The reaction mixture was cooled to 25° and 1.5 ml of distilled water was added to the mixture before measurement of the absorbance at 540 nm (Genesys 20 4001/4; ThermoFisher Scientific, USA). Using a maltose standard curve and absorbance of sample, the amount of reducing sugars released was calculated and converted to reaction velocity. The double reciprocal plot or Lineweaver-Burk plot of 1/[S] against 1/V where V is reaction Velocity and [S] is Substrate (starch) concentration was constructed. Mode of inhibition can be obtained from the graph analysis.
Statistical analysis:
All experiments were performed in triplicates. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) software ver. 25.0 (IBM, Armonk, New York, USA). Data were subjected to Analysis of Variance (ANOVA). Duncan’s new multiple range test was used to evaluate the significant differences between means and a value of p<0.05 was considered as statistically significant. Values of 1/IC50 and other data were used in correlation analysis and the results were reported as the Pearson’s correlation coefficients (r).
Results and Discussion
Phytochemical contents among AAE and its fractions (EF, WF, AMF and AAF) were significantly different (Table 1). TPC in AAE and its fractions expressed as mg GAE/g DW significantly decreased in the order of EF>AAF>AAE>WF>AMF while TPAC expressed as mg CE/g DW decreased in the order of AAF>AAE>WF>EF>AMF.
| Sample | TPC (mg GAE/g DW) | TPAC (mg CE/g DW) |
|---|---|---|
| AAE | 288.10±0.73c | 333.35±0.96d |
| EF | 495.68±0.00e | 121.92±0.24b |
| WF | 268.17±0.24b | 329.41±2.08c |
| AMF | 86.82±0.12a | 22.02±0.06a |
| AAF | 475.05±0.47d | 536.11±4.15e |
Note: The results are expressed as mean±Standard Deviation (SD) (n=3). (a-e)Different letters in the same column are significantly different at p<0.05; AAE: Aqueous Acetone Extract; EF: Ethyl Acetate Fraction; WF: Water-Soluble Fraction; AMF: Aqueous Methanol Fraction and AAF: Aqueous Acetone Fraction
Table 1: TPC and TPAC of AAE and its Fractions from A. Martini Root.
AAE and its fractions and standards (BHT,ascorbicacidand Trolox) showed a linear relationship between percentage of free radical inhibition and sample concentrations in DPPH and ABTS assays (data not shown). Each IC50 value calculated using the linear equation is shown in Table 2. IC50 is the sample concentration required to cause 50 % inhibition; therefore, lower IC50 values indicate greater radical scavenging activity[23,27]. For the DPPH assay, calculated IC50 values revealed that AAE and its fractions had significantly higher inhibition of DPPH radicals compared to BHT. AAF had the highest antioxidant activity as revealed by the lowest IC50 and antioxidant activity of AAF was much higher than that of Trolox, but similar to that of ascorbic acid. For the ABTS assay, according to their IC50 values, the scavenging effect on the ABTS radicals decreased in the order of AAF>EF>AAE>WF>AMF. All extracts were determined to have weaker antioxidant activities than ascorbic acid. However, AAF had more efficient antioxidant activity than standards (BHT and Trolox).
| Sample | DPPH IC50 (µg/ml) | ABTS IC50 (mg/ml) | CUPRAC (mg TE/g DW) | FRAP (mmol Fe2+/g DW) |
|---|---|---|---|---|
| AAE | 17.80±0.02d | 11.34±0.03f | 994.79±1.73c | 311.42±0.39d |
| EF | 35.00±0.06e | 10.53±0.02e | 685.73±0.65b | 162.38±0.34c |
| WF | 16.36±0.02c | 11.46±0.04g | 1023.03±1.73d | 388.66±0.39e |
| AMF | 50.58±0.12f | 45.22±0.06h | 220.88±0.54a | 101.67±0.32a |
| AAF | 7.12±0.00a | 6.27±0.01b | 2027.95±8.81f | 773.50±4.67g |
| BHT | 83.46±0.80g | 7.52±0.03c | 685.38±2.62b | 132.73±0.69b |
| Ascorbic acid | 6.91±0.02a | 5.64±0.01a | 1757.58±4.34e | 1039.25±6.44h |
| Trolox | 13.79±0.05b | 8.29±0.01d | ND | 728.38±3.58f |
Note: The results are expressed as mean±SD (n=3) which, with (a-h)different letters in the same column are significantly different at p<0.05. IC50 is the Inhibitory Concentration of sample to scavenge 50 % radicals. ND: Not Determined; AAE: Aqueous Acetone Extract; EF: Ethyl Acetate Fraction; WF: Water-Soluble Fraction; AMF: Aqueous Methanol Fraction; AAF: Aqueous Acetone Fraction; DW: Dry Weight; DPPH: 2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate; ABTS: 2,2'-Azino-bis(3-Ethylbenzothiazoline-6-Sulfonic acid); CUPRAC: Cupric Reducing Antioxidant Capacity and FRAP: Ferric Reducing Antioxidant Power
Table 2: Antioxidant Activities of AAE and its Fractions from A. Martini root
The antioxidant activity can also be expressed through measurement of the reduction of metal ions such as Cupric ion (Cu2+) and Ferric ion (Fe3+) by antioxidants in the sample. For the CUPRAC assay, copper (II)- neocuproine [Cu(II)-Nc] complex was reduced to form the Cu(I)-Nc[26] while for the FRAP assay, ferric tripyridyltriazine [Fe(III)-TPTZ] complex was reduced to form the ferrous tripyridyltriazine [Fe(II)-TPTZ] complex[27]. High CUPRAC and FRAP values indicate high antioxidant activity. As shown in Table 2, AAF demonstrated the highest antioxidant activity followed by WF>AAE>EF>AMF, respectively in both assays. In addition, AAF showed much higher antioxidant activity compared to ascorbic acid and BHT in CUPRAC assay and had significantly higher antioxidant activity compared to BHT and Trolox in FRAP assay.
Acarbose and all samples except AMF inhibited α-amylase in a dose-dependent manner (data not shown). IC50 (sample concentration causing 50 % enzyme inhibition) of AAE and its fractions was found to range from 8.77±0.28 µg/ml to 21.36±0.30 µg/ml while IC50 of acarbose was found to be 21.19±0.79 µg/ml (Table 3). AAF showed the lowest IC50 value; therefore, AAF had much higher α-amylase inhibitory activity compared to other samples and standard acarbose. There was no detectable activity in AMF, therefore, its IC50 value could not be calculated.
| Sample | Linear regression equation | R2 | IC50 (µg/ml) |
|---|---|---|---|
| AAE | y=5.1689x-8.9881 | 0.9898 | 11.41±0.1b |
| EF | y=1.1158x+30.819 | 0.9849 | 17.18±0.49c |
| WF | y=2.5401x-4.2417 | 0.9777 | 21.36±0.30d |
| AMF | - | - | n. i. |
| AAF | y=5.9287x-1.9497 | 0.9860 | 8.77±0.28a |
| Acarbose | y=1.1409x+25.867 | 0.9866 | 21.19±0.79d |
Note: IC50: Amount required for a 50 % inhibition of α-amylase activity. The results are expressed as mean±SD (n=3) which, with (a-d)different letters in the same column are significantly different at p<0.05. n. i.: No inhibition observed up to 100 µg/ml, -: No data; AAE: Aqueous Acetone Extract; EF: Ethyl Acetate Fraction; WF: Water-Soluble Fraction; AMF: Aqueous Methanol Fraction and AAF: Aqueous Acetone Fraction
Table 3: Linear Regression Equation, R2 and IC50 for α-Amylase Inhibition of AAE and its Fractions from A. Martini root
Kinetic study employed the Lineweaver-Burk plot to identify the mode of α-amylase inhibition by inhibitor. AAF showing the lowest IC50 value for α-amylase inhibition which was tested in this study. AAF displayed a mixed noncompetitive mode of inhibition towards α-amylase (fig. 1A) while acarbose showed noncompetitive inhibition on the enzyme (fig. 1B).
Pearson’s correlation analysis was performed to test the possible relationship between phytochemicals, antioxidant activity and α-amylase inhibitory activity in A. martini roots. The results were reported as Pearson’s correlation coefficient (r) and shown in Table 4. α-Amylase inhibitory activity was significantly correlated with TPC (r=0.748), TPAC (r=0.878) and all antioxidant activities (r≥0.804). In addition, TPAC showed higher correlation to all antioxidant activities compared to TPC.
| Assay | TPC | TPAC | DPPH | ABTS | CUPRAC | FRAP | Amylase inhibition |
|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.503 | 0.511 | 0.846** | 0.639* | 0.506 | 0.748** |
| TPAC | 1 | 0.935** | 0.877** | 0.963** | 0.950** | 0.878** | |
| DPPH | 1 | 0.880** | 0.980** | 0.996** | 0.815** | ||
| ABTS | 1 | 0.950** | 0.881** | 0.927** | |||
| CUPRAC | 1 | 0.982** | 0.895** | ||||
| FRAP | 1 | 0.804** | |||||
| Amylase inhibition | 1 |
Note: *Correlation is significant at the 0.05 level and **Correlation is significant at the 0.01 level
Table 4: Pearson’s Correlation Coefficient of Phytochemical Contents, Antioxidant Activity and α-Amylase Inhibition
Using natural phenolic compounds including proanthocyanidins which has α-amylase inhibitory activity and strong antioxidant activity is an effective treatment of type 2 diabetes[3,6,14,15]. Therefore, TPC, TPAC, antioxidant activity, α-amylase inhibitory activity and their relation were evaluated in AAE and its fractions (EF, WF, AMF and AAF) from A. martini root.
EF and AAF showed the highest TPC and TPAC, respectively. Difference in TPC and TPAC found in AAE and its fractions may have resulted from many factors such as chemical structures of phenolic compounds including proanthocyanidins, polarity of different solvents used, extraction methods and the presence of interfering substances[31]. Phenolic compounds including proanthocyanidins have diverse structures resulting in different solubilities of the compounds in water and organic solvents[13]. AAE was obtained from 70 % aqueous acetone, which is a commonly used solvent system for extraction of phenolic compounds and proanthocyanidins[32,33]. Using ethyl acetate (which has lower polarity compared to water) helps to remove low molecular weight phenolics including monomeric flavonoids and partly oligomeric proanthocyanidins from the aqueous fraction (WF)[34] and yields EF. In fractionation of WF by Sephadex LH-20 column chromatography, aqueous methanol (50 % v/v) has been used to remove polysaccharides and some monomeric flavonoids from the column before elution with aqueous acetone (70 % v/v) was performed to obtain concentrated polymeric proanthocyanidins[22,34].
Several methods such as DPPH, ABTS, CUPRAC and FRAP were performed to evaluate the antioxidant activity of substances by the reduction of certain compounds including metals and radicals because they are simple and widely used techniques[10,13]. AAF showed higher antioxidant activity compared to all samples in all assays. Correlation analysis confirmed that the strongest antioxidant activity of AAF was related to high proanthocyanidin content (Table 4). The results were consistent with the report of Lin et al.[35] demonstrating that antioxidant activity had high correlation coefficient with proanthocyanidin content. Phenolic compounds especially proanthocyanidins are structurally diverse. The number and positions of the hydroxyl groups, and the nature of substitutions on the aromatic rings give rise to the scavenging free radicals, donating hydrogen atoms or electrons, or chelating metal cations[36]. AAF, proanthocyanidin-rich fraction showed stronger antioxidant activity compared to two standards (BHT, trolox) in both free radical scavenging activity and metal reducing power; therefore, it might be used as a source of alternative antioxidant.
Using inhibition of α-amylase has been a therapeutic approach for controlling postprandial hyperglycemia in type 2 diabetes because it decreases the rate of starch degradation, which would in turn causes decreasing glucose absorption and concentration of postprandial blood glucose[6,37]. AAF showed higher α-amylase inhibitory activity compared to all samples. This might have resulted from high content of TPC and TPAC because correlation analysis showed that α-amylase inhibitory activity was significantly correlated with TPC (r=0.748), TPAC (r=0.878) (Table 4). No detectable activity in AMF may have resulted from the low values of both TPC and TPAC (Table 1). The results were consistent with the report of Lin et al.[35] demonstrating that the α-amylase inhibitory activity had a high correlation coefficient with proanthocyanidin content. Also, a significant correlation between the phenolic content and amylase inhibitory activity has been observed in other reports[38,39]. Phenolic compounds including proanthocyanidins can inhibit α-amylase by cooperative effects of hydrophobic interaction and hydrogen bond formation between the substances and the enzyme[40]. In addition, AAF acted as mixed noncompetitive inhibitor of the enzyme. Mixed noncompetitive inhibitor can bind to either the free enzyme or the Enzyme-Substrate (ES) complex and a single inhibitor can prevent the binding of substrate and decreases the turnover number of the enzyme[41].
In conclusion, this study confirms that A. martini root can be a good source of natural phytochemicals and that there were correlations among contents of phenolics and proanthocyanidins, antioxidant and α-amylase inhibitory activities in AAE and its fractions from the plant root. Proanthocyanidins had strong correlation with both α-amylase inhibitory and antioxidant activities; therefore, AAF, proanthocyanidins-rich fraction, might be used for effective diabetes management. Further in vivo assays for both activities as well as proanthocyanidin structure identification in AAF may be required to advance the study.
Acknowledgements:
This research was financially supported by the Faculty of Science, Mahasarakham University (Grant year 2020), Thailand. The author acknowledges Associate Professor, Dr. Prasong Srihanam of Faculty of Science, Mahasarakham University for source of A. martini root and Dr. Khanit Wangwasit, Department of Biology, Faculty of Science, Mahasarakham University, Thailand for help in the identification and authentication of A.martini specimen.
Conflict of interests:
The authors declared no conflict of interest.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(1):S62-9.
- Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia?induced free radical generation leading to oxidative stress. J Cell Physiol 2019;234(2):1300-12.
[Crossref] [Google scholar] [PubMed]
- Apostolidis E, Li L, Lee C, Seeram NP. In vitro evaluation of phenolic-enriched maple syrup extracts for inhibition of carbohydrate hydrolyzing enzymes relevant to type 2 diabetes management. J Funct Foods 2011;3(2):100-6.
- Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc Diabetol 2005;4(1):1-11.
[Crossref] [Google scholar] [PubMed]
- Makinde EA, Ovatlarnporn C, Adekoya AE, Nwabor OF, Olatunji OJ. Antidiabetic, antioxidant and antimicrobial activity of the aerial part of Tiliacora triandra. S Afr J Bot 2019;125:337-43.
- Oboh G, Isaac AT, Akinyemi AJ, Ajani RA. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int J Biomed Sci 2014;10(3):208-16.
[Google scholar] [PubMed]
- Oboh G, Ogunsuyi OB, Ogunbadejo MD, Adefegha SA. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J Food Drug Anal 2016;24(3):627-34.
[Crossref] [Google scholar] [PubMed]
- Ademiluyi AO, Oboh G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp Toxicol Pathol 2013;65(3):305-9.
[Crossref] [Google scholar] [PubMed]
- Oboh G, Ademosun AO, Odubanjo OV, Akinbola IA. Antioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepper. Adv Pharmacol Pharm Sci 2013;2013:1-6.
[Crossref] [Google scholar] [PubMed]
- Hieu HV, Tatipamula VB, Killari KN, Koneru ST, Srilakshmi N, Ranajith SK. HPTLC analysis, antioxidant and antidiabetic activities of ethanol extract of moss Fissidens grandiflora. Indian J Pharm Sci 2020;82(3):449-55.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2(5):270-8.
[Crossref] [Google scholar] [PubMed]
- Aryaeian N, Sedehi SK, Arablou T. Polyphenols and their effects on diabetes management: A review. Med J Islam Repub Iran 2017;31:134.
[Crossref] [Google scholar] [PubMed]
- san Miguel-Chavez R. Phenolic antioxidant capacity: A review of the state of the art. In: Soto-Hernandez, M, Palma-Tenango M, Garcia-Mateos MdR, editors. Phenolic compounds-Biological activity. Rijeka: IntechOpen; 2017.p. 59-74.
- Lee YA, Cho EJ, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J Nutr Sci Vitaminol 2007;53(3):287-92.
[Crossref] [Google scholar] [PubMed]
- Anh H, Xuan TD, NT DT, Quan NV, Trang LT. Antioxidant and a-amylase inhibitory activities and phytocompounds of Clausena indica Fruits. Medicines 2020;7(3):10.
[Crossref] [Google scholar] [PubMed]
- Yardpiroon B, Aphidech S, Prasong S. Phytochemical and biological activities of the wild grape fruit extracts using different solvents. J Pharm Res Int 2014;4:23-36.
- Vittaya L, Aiamyang S, Ui-eng J, Khongsai S, Leesakul N. Effect of solvent extraction on phytochemical component and antioxidant activity of vine and rhizome Ampelocissusmartini. Sci Technol Asia 2019;24:17-26.
- Siripipatthana P, Srihanam P. Fractionation and identification of phytochemicals and antioxidant activity of wild grape (Ampelocissus martini Planch.) seed extracts. Asian J Chem 2020;32(7):1703-7.
- Jirum J, Sangdee A, Srihanam P. Phytochemical and biological activities in fresh juice extracts of wild grape (Ampelocissus martini Planch). Fruits. Int J Res Ayurveda Pharm 2013;4(3):337-41.
- Chen XX, Shi Y, Chai WM, Feng HL, Zhuang JX, Chen QX. Condensed tannins from Ficus virens as tyrosinase inhibitors: Structure, inhibitory activity and molecular mechanism. PLoS One 2014;9(3):e91809.
[Crossref] [Google scholar] [PubMed]
- Zhou HC, Lin YM, Wei SD, Tam NF. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem 2011;129(4):1710-20.
- Chai WM, Lin MZ, Feng HL, Zou ZR, Wang YX. Proanthocyanidins purified from fruit pericarp of Clausena lansium (Lour.) Skeels as efficient tyrosinase inhibitors: Structure evaluation, inhibitory activity and molecular mechanism. Food Funct 2017;8(3):1043-51.
- Farhadi K, Esmaeilzadeh F, Hatami M, Forough M, Molaie R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem 2016;199:847-55.
[Crossref] [Google scholar] [PubMed]
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 2006;96(2):254-60.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26(9):1231-7.
- Apak R, Güçlü K, Özyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 2004;52(26):7970-81.
[Crossref] [Google scholar] [PubMed]
- Zhang LL, Lin YM. Tannins from Canarium album with potent antioxidant activity. J Zhejiang Univ Sci B 2008;9(5):407-15.
[Crossref] [Google scholar] [PubMed]
- Wickramaratne MN, Punchihewa JC, Wickramaratne DB. In vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern Med 2016;16(1):1-5.
[Crossref] [Google scholar] [PubMed]
- Olaokun OO, Alaba AE, Ligege K, Mkolo NM. Phytochemical content, antidiabetes, anti-inflammatory antioxidant and cytotoxic activity of leaf extracts of Elephantorrhiza elephantina (Burch.) Skeels. S Afr J Bot 2020;128:319-25.
- Kazeem MI, Adamson JO, Ogunwande IA. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. Biomed Res Int 2013;2013:1-6.
[Crossref] [Google scholar] [PubMed]
- Abu F, Mat Taib CN, Mohd Moklas MA, Mohd Akhir S. Antioxidant properties of crude extract, partition extract and fermented medium of Dendrobium sabin flower. Evid Based Complement Alternat Med 2017;2017:1-9.
[Crossref] [Google scholar] [PubMed]
- Zuorro A, Iannone A, Lavecchia R. Water-organic solvent extraction of phenolic antioxidants from brewers’ spent grain. Processes 2019;7(3):126.
- Downey MO, Hanlin RL. Comparison of ethanol and acetone mixtures for extraction of condensed tannin from grape skin. South African J Enol Vitic 2010;31(2):154-9.
- Ku CS, Mun SP. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci Technol 2007;41(3):235-47.
- Lin GM, Chen YH, Yen PL, Chang ST. Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. J Tradit Complement Med 2016;6(3):281-8.
[Crossref] [Google scholar] [PubMed]
- Minatel IO, Borges CV, Fereira MI, Gomez HAG, Chen CYO, Lima GPP. Phenolic compounds: Functional properties, impact of processing and bioavailability. In: Soto-Hernandez M, Palma-Tenango M, Garcia-Mateos MdR, editors. Phenolic compounds-biological activity. Rijeka: IntechOpen; 2017. p. 1-24.
- Balan K, Ratha P, Prakash G, Viswanathamurthi P, Adisakwattana S, Palvannan T. Evaluation of in vitro α-amylase and α-glucosidase inhibitory potential of N2O2 schiff base Zn complex. Arab J Chem 2017;10(5):732-8.
- Rana ZH, Alam MK, Akhtaruzzaman M. Nutritional composition, total phenolic content, antioxidant and α-amylase inhibitory activities of different fractions of selected wild edible plants. Antioxidants 2019;8(7):203.
[Crossref] [Google scholar] [PubMed]
- Sarikurkcu C, Kakouri E, Sarikurkcu RT, Tarantilis PA. Study on the chemical composition, enzyme inhibition and antioxidant activity of Ziziphora taurica subsp. cleonioides. Appl Sci 2019;9(24):5515.
- Ali Asgar MD. Anti-diabetic potential of phenolic compounds: A review. Int J Food Prop 2013;16(1):91-103.
- Berg JM, Tymoczko JL, Stryer L. Biochemistry 5th ed. New York: W.H. Freeman and Co Ltd; 2002.