- *Corresponding Author:
- Abinaya Chinnadurai
Department of Medicinal and Aromatic Crops, Tamil Nadu Agricultural University, Coimbatore 641003, Tamil Nadu, India
E-mail: abi.abinaya177@gmail.com
| Date of Received | 01 November 2023 |
| Date of Revision | 03 May 2024 |
| Date of Acceptance | 24 October 2024 |
| Indian J Pharm Sci 2024;86(5):1756-1764 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Aloe vera, historically acclaimed as the plant of immortality, has gained significant attention for its therapeutic potential, particularly in dermatology and anti-inflammatory applications. In this study, we use a computational approach that integrates molecular docking analysis and pharmacokinetic assessment to explore the inhibitory potential of aloe vera phytochemicals against tumor necrosis factoralpha, a key inflammatory protein implicated in various pathologies, including rheumatoid arthritis. The in silico screening identified top-performing compounds, with aloesin emerging as a promising inhibitor. Pharmacological analyses revealed aloesin's favourable toxicity profile, positioning it as a promising candidate for further drug development. This research provides insights into the anti-inflammatory properties of aloesin in Aloe vera, paving the way for future experimental validations and the development of novel therapeutics for rheumatoid arthritis.
Keywords
Aloe vera, anti-inflammatory, molecular docking, aloesin, pharmacokinetics
A plant product plays a crucial role in combating human diseases. They contain a variety of bioactive compounds that offer therapeutic benefits through multiple mechanisms. These include antioxidant and anti-inflammatory properties, antimicrobial activities, and immune system modulation[1]. Many plant derived substances also target specific biological pathways involved in disease processes. Furthermore, plant products serve as important sources for novel drug development in modern medicine. Their complex composition often provides synergistic effects, enhancing their overall therapeutic potential against various human diseases. Aloe vera, recognized for its historical significance as the plant of immortality, holds profound pharmaceutical importance in both traditional and modern medicine. Its gel, containing a diverse array of bioactive compounds, positions aloe vera as a versatile candidate for therapeutic applications, particularly in dermatological formulations, wound healing, and anti-inflammatory interventions. The increasing global demand for natural remedies has driven a substantial rise in aloe vera production and cultivation. Its prevalence spans across arid regions worldwide, contributing significantly to the pharmaceutical and cosmetic industries. Noteworthy cultivation areas include India, Australia, United States of America (USA), Japan, and Europe, attesting to its economic importance and widespread applications. Inflammation, a pivotal aspect of the body's immune response, is intricately regulated by molecular signalling pathways. Tumour Necrosis Factor (TNF), a key inflammatory protein, plays a key role in initiating and perpetuating inflammatory cascades, implicated in various pathological conditions. Rheumatoid Arthritis (RA), a systemic autoimmune disorder, remains a challenge in medical treatment despite current interventions such as Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Disease-Modifying Anti-Rheumatic Drugs (DMARDs), specifically TNF-Alpha (α) inhibitors.
Aloe vera, renowned for its medicinal properties, has garnered scientific interest for its potential anti- inflammatory effects. Aloe vera’s polysaccharides, phenolic compounds, and sterols have emerged as key players in modulating inflammatory pathways. This study employs an in silico approach, utilizing molecular docking analyses and pharmacokinetic assessments, to evaluate the inhibitory potential of aloe phytochemicals against TNF-α. This computational analysis aims to provide insights into the therapeutic efficacy of aloe vera constituents against TNF-α, offering a foundation for future experimental investigations.
Materials and Methods
Selection of phytochemical compounds in aloe vera and anti-inflammatory drugs:
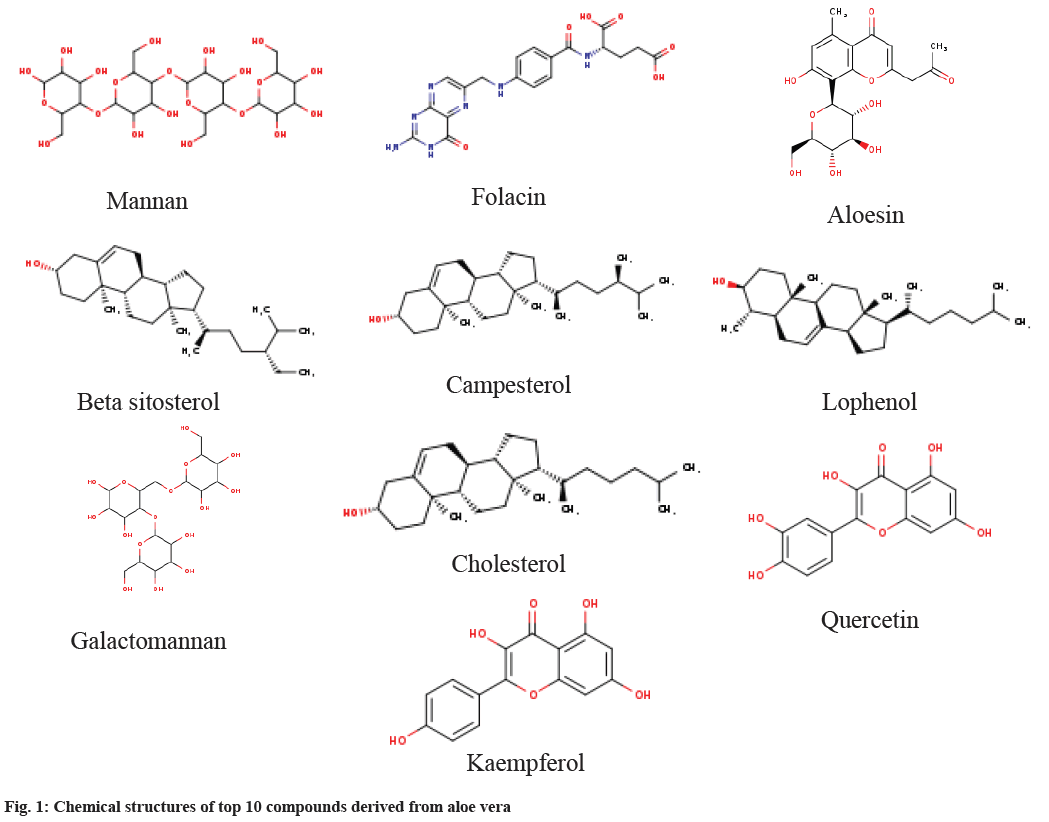
A curated selection of aloe vera phytochemicals was undertaken through a comprehensive literature review and utilization of the Indian Medicinal Plants, Phytochemistry And Therapeutics (IMPPAT) (https://cb.imsc.res.in/imppat) and ZINC databases. Phytochemicals were chosen based on documented presence in the literature and availability within these databases, ensuring a systematic approach aligned with established knowledge. Approved anti-inflammatory drugs from the therapeutic target database served as benchmarks for comparing the efficacy of bioactive compounds derived from aloe vera. This approach aimed to assess the relative effectiveness of aloe vera compounds against established anti-inflammatory medications (fig. 1).
Molecular docking studies:
The primary objective in molecular docking was to accurately gauge the scoring function and assess interactions between proteins and ligands. AutoDock Vina, coupled with PyRx, was employed for generating a dataset of bioactive binding poses of ligands within the active site of TNF-α. Additionally, the Discovery Studio 2024 client software was utilized to model nonbonded polar and hydrophobic contacts within the inhibitor site of TNF-α[2].
Structure retrieval of compounds:
Three-dimensional structures of bioactive compounds in aloe vera and over-the-counter medicines recommended for inflammation, such as aspirin, hydrocortisone, melaxicam, ibuprofen, celecoxib, and naproxen, along with the three-dimensional structures of the target protein TNF-α, were retrieved from databases such as the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB) and PubChem.
Molecular docking software:
Molecular docking studies were conducted using PyRx, an open-source tool that employs an iterative approach to predict ligand poses within the protein's binding site, and Discovery Studio, a comprehensive molecular modeling and simulation software integrating various algorithms and scoring functions for accurate prediction of protein-ligand binding affinities.
Docking procedure:
Preparation of protein: The three-dimensional structure of TNF obtained from RCSB PDB was processed to remove water molecules and optimize hydrogen bonds.
Ligand preparation: Aloe vera compounds and commercial drug compounds retrieved from PubChem were prepared by assigning correct bond orders and optimizing conformations. Ligands were loaded into PyRx virtual screening software using Open Babel for conversion to Program Database (PDB) format.
Grid generation: Docking was performed using AutoDock Vina in PyRx virtual screening software with specific grid parameters. The Lamarckian Genetic Algorithm (LGA) was employed to generate 10 docked positions for each ligand. Subsequently, docking results were analyzed and visualized based on docking scores using Discovery Studio 2024 client and PyMOL software.
Scoring and analysis:
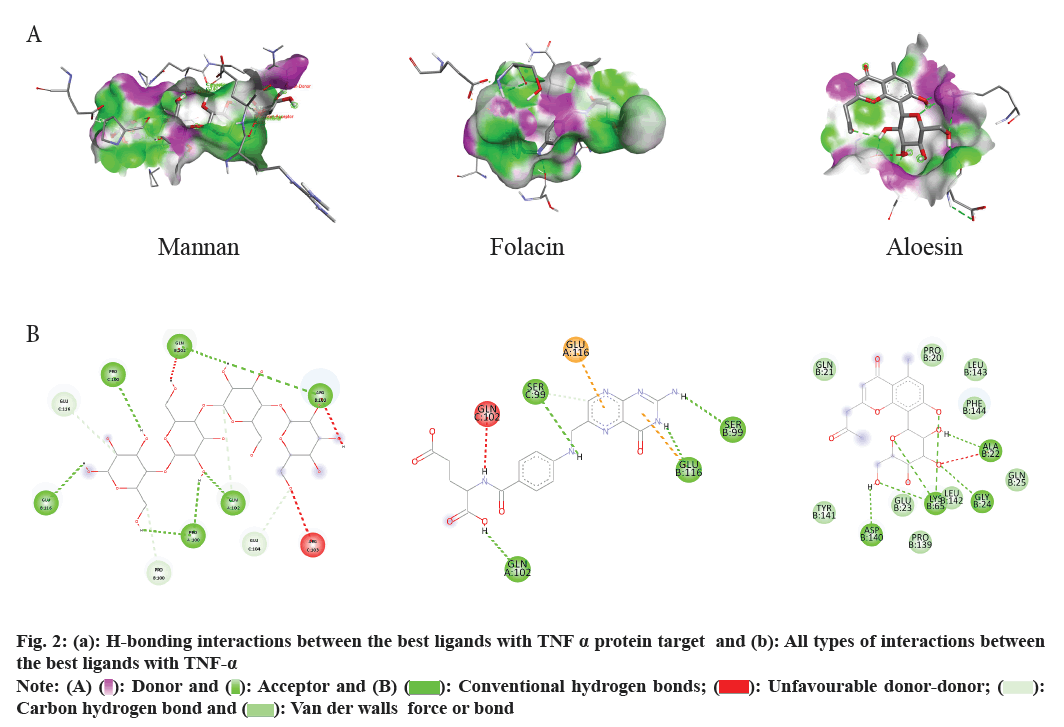
Docking scores, binding efficiency, and hydrogen bond interactions were analyzed to evaluate the strength and specificity of ligand binding. Molecular docking results were thoroughly analyzed for binding affinity, and the most promising aloe vera compound was selected based on docking scores and hydrogen bond interactions with TNF (fig. 2a and fig. 2b).
Fig. 2: (a): H-bonding interactions between the best ligands with TNF a protein target and (b): All types of interactions between
the best ligands with TNF-a Note: (A)  Donor and
Donor and  Acceptor and (B)
Acceptor and (B)  Conventional hydrogen bonds;
Conventional hydrogen bonds;  Unfavourable donor-donor;
Unfavourable donor-donor;  Carbon hydrogen bond and
Carbon hydrogen bond and  Van der walls force or bond
Van der walls force or bond
Pharmacology analysis:
Pharmacokinetic analysis of ligands was systematically performed using the SwissADME server (http://www.swissadme.ch/). This computational tool facilitated the comprehensive evaluation of various pharmacokinetic parameters, encompassing absorption, metabolism, distribution, excretion, and toxicity predictions for the prospective compounds under investigation. Additionally, the SwissADME server provided insights into critical aspects such as bioavailability score, druggability, and synthetic accessibility score, offering a holistic perspective on the pharmaceutical viability of the examined compounds. Ligands were screened according to Lipinski's Rule of Five (RO5), for safety assessment in drug development, and toxicology predictions were performed using the Small-molecule pharmacokinetics prediction (pkCSM) online server. Parameters analyzed included Ames toxicity, maximum tolerance dose, human Ether-a- go-go Related Gene (hERG) inhibition, Lethal Dose 50 (LD50), Lowest Observed Adverse Effect Level (LOAEL), hepatotoxicity, skin toxicity, Tetrahymena pyriformis toxicity, and minnow toxicity. This rigorous pharmacological scrutiny aims to inform and guide further exploration of these compounds in drug development endeavours.
Results and Discussion
The study addresses the considerable socio-economic burden posed by the inflammatory autoimmune disease rheumatoid arthritis, affecting around 1 % of the global population[3,4]. Acknowledging the limitations of current RA treatments, the research aims to explore alternative therapeutic interventions with reduced side effects. Aloe vera demonstrates anti-inflammatory properties by effectively inhibiting the cyclooxygenase pathway, resulting in a diminished synthesis of prostaglandins and, consequently, a reduction in inflammatory processes[5-7]. The bioactive compounds in aloe vera demonstrate an inhibitory effect on the release of pro-inflammatory mediators, such as cytokines and histamine[8,9]. The principal focus of the study was to investigate the potential of phytochemicals to target the cytokine TNF-α, thereby demonstrating their anti-inflammatory activity. In pursuit of identifying active chemical constituents of aloe vera possessing potential interactions with the TNF-α protein, molecular docking studies were conducted for a set of 74 aloe vera chemical constituents (Table 1).
| S.No. | Ligand | PubChem ID | Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|
| 1 | 2(3H)-Benzothiazolone | 13625 | C7H5NOS | 151.19 |
| 2 | 3,4-Dihydrocoumarin | 660 | C9H8O2 | 148.16 |
| 3 | 7-Hydroxy-4-benzopyrone | 5409279 | C9H6O3 | 162.14 |
| 4 | 15-Methylhexadecanoic acid | 164860 | C17H34O2 | 270.5 |
| 5 | Acemannan | 72041 | C66H100NO49 | 1691.5 |
| 6 | Allantoin | 204 | C4H6N4O3 | 158.12 |
| 7 | Aloe emodin | 10207 | C15H10O5 | 270.24 |
| 8 | Aloenin | 162305 | C19H22O10 | 410.4 |
| 9 | Aloeresin | 160190 | C19H22O9 | 394.4 |
| 10 | Aloesone | 5317700 | C13H12O4 | 232.23 |
| 11 | Aloin A | 12305761 | C21H22O9 | 418.4 |
| 12 | Aloin B | 14989 | C21H22O9 | 418.4 |
| 13 | Aluminum | 5359268 | Al | 26.981 |
| 14 | anthracene | 8418 | C14H10 | 178.23 |
| 15 | anthranol | 10731 | C14H10O | 194.23 |
| 16 | Anthraquinone | 6780 | C14H8O2 | 208.21 |
| 17 | Ascorbic Acid | 54670067 | C6H8O6 | 176.12 |
| 18 | Asparagine | 6267 | C4H8N2O3 | 132.12 |
| 19 | Aspirin | 2244 | C9H8O4 | 180.16 |
| 20 | Auxin | 802 | C10H9NO2 | 175.18 |
| 21 | beta carotene | 5280489 | C40H56 | 536.9 |
| 22 | beta sitosterol | 222284 | C29H50O | 414.7 |
| 23 | Campesterol | 173183 | C28H48O | 400.7 |
| 24 | Carvacrol | 10364 | C10H14O | 150.22 |
| 25 | Caryophyllene oxide | 1742210 | C15H24O | 220.35 |
| 26 | Caryophyllene | 5281515 | C15H24 | 204.35 |
| 27 | Celecoxib | 2662 | C17H14F3N3O2S | 381.4 |
| 28 | Cholesterol | 5997 | C27H46O | 386.7 |
| 29 | Chrysophanic acid | 10208 | C15H10O4 | 254.24 |
| 30 | chrysophanol | 10208 | C15H10O4 | 254.24 |
| 31 | Citric acid | 311 | C6H8O7 | 192.12 |
| 32 | Creatinine | 588 | C4H7N3O | 113.12 |
| 33 | Cycloartenol | 92110 | C30H50O | 426.7 |
| 34 | Cysteine hydrochloride | 25150 | C3H8ClNO2S | 157.62 |
| 35 | Cysteine | 5862 | C3H7NO2S | 121.16 |
| 36 | D fructose | 2723872 | C6H12O6 | 180.16 |
| 37 | D galactonic acid | 128869 | C6H12O7 | 196.16 |
| 38 | D galactose | 6036 | C6H12O6 | 180.16 |
| 39 | D glucose | 5793 | C6H12O6 | 180.16 |
| 40 | D mannose | 18950 | C6H12O6 | 180.16 |
| 41 | d Tartaric acid | 439655 | C4H6O6 | 150.09 |
| 42 | Danshenxinkun A | 149138 | C18H16O4 | 296.3 |
| 43 | Danthron | 2950 | C14H8O4 | 240.21 |
| 44 | Docosane | 12405 | C22H46 | 310.6 |
| 45 | elgonica dimer A | 21582596 | C36H30O14 | 686.6 |
| 46 | Feralolide | 5317333 | C18H16O7 | 344.3 |
| 47 | Folacin | 135398658 | C19H19N7O6 | 441.4 |
| 48 | Galactomannan | 439336 | C18H32O16 | 504.4 |
| 49 | Globulin G | 74329879 | C36H61N7O19 | 895.9 |
| 50 | Hydrocortisone | 5754 | C21H30O5 | 362.5 |
| 51 | Ibuprofen | 3672 | C13H18O2 | 206.28 |
| 52 | Isoaloeresin | 76332505 | C29H32O11 | 556.6 |
| 53 | kaempferol | 5280863 | C15H10O6 | 286.24 |
| 54 | L Arabinose | 439195 | C5H10O5 | 150.13 |
| 55 | Leucine | 6106 | C6H13NO2 | 131.17 |
| 56 | linalool | 6549 | C10H18O | 154.25 |
| 57 | Lophenol | 160482 | C28H48O | 400.7 |
| 58 | Lupeol | 259846 | C30H50O | 426.7 |
| 59 | Mannan | 25147451 | C24H42O21 | 666.6 |
| 60 | Meloxicam | 54677470 | C14H13N3O4S2 | 351.4 |
| 61 | Naproxen | 156391 | C14H14O3 | 230.26 |
| 62 | Niacin | 938 | C6H5NO2 | 123.11 |
| 63 | Phenylalanine | 6140 | C9H11NO2 | 165.19 |
| 64 | Potassium | 5462222 | K | 39.098 |
| 65 | Proline | 145742 | C5H9NO2 | 115.13 |
| 66 | Quercetin | 5280343 | C15H10O7 | 302.23 |
| 67 | Rhababerone | 12310964 | C15H10O5 | 270.24 |
| 68 | Salicylic acid | 338 | C7H6O3 | 138.12 |
| 69 | Serine | 5951 | C3H7NO3 | 105.09 |
| 70 | Sorbitol | 5780 | C6H14O6 | 182.17 |
| 71 | Spathulenol | 92231 | C15H24O | 220.35 |
| 72 | Threonine | 6288 | C4H9NO3 | 119.12 |
| 73 | Thymol acetate | 68252 | C12H16O2 | 192.25 |
| 74 | Tricosane | 12534 | C23H48 | 324.6 |
Table 1: Characteristic of Phytoconstituents of Aloe Vera
The use of AutoDock Vina facilitated the determination of molecular interactions and binding energy between aloe vera phytoconstituents and TNF-α protein, contributing valuable insights for drug discovery endeavours. Molecular docking revealed significant interactions between aloe vera compounds and TNF-α. The measure of the affinity in a ligand-protein complex is termed as binding energy. It represents the difference between the energy of the complex (ligand bound to the protein) and the sum of the energies of each molecule separately (ligand and protein considered as independent entities). In other words, it quantifies the stability and strength of the interaction between the ligand and the protein in a molecular complex. A lower binding energy typically indicates a more favourable and stronger binding interaction. Table 2 summarizes the binding energies and key interactions of the top-performing compounds, thus revealing several high-energy interactions between aloe vera compounds and TNF-α. The observed binding affinity values range from -10 to -9.1 kcal/mol. Mannan exhibited the highest binding affinity with a docking score of -10.0 kcal/mol, forming multiple hydrogen bonds with PRO 100C, Glutamine (GLN) 102B, Arginine (ARG) 103B, Glutamine (GLN) 102A, PRO 100A, and Glutamic acid (GLU) 116B. It also engaged in hydrophobic interactions with GLU 116C, ARG 103A, GLU 104C, and PRO 100B.
| Compound name | Docking score (Kcal/mol) | Amino acids with hydrogen bonds | Amino acids with hydrophobic interactions |
|---|---|---|---|
| Drug references | |||
| Aspirin | -5.6 | GLN 102B | GLN 102A |
| Celecoxib | -7.6 | ARG 103B, GLU 104B | GLN 102A, GLN 102B, GLN 102C, GLU 104A |
| Hydrocortisone | -6.3 | ALN 33A, ASN 34A, ARG 82C | |
| Ibuprufen | -6.5 | TYR 115A, SER 99C, CYS 101A | PRO 100A |
| Meloxicam | -6.3 | ARG 103C, GLU 104A, GLU 104B, GLU 104C, GLN 102B, ARG 103A | ARG 103B |
| Naproxen | -7.1 | LYS 65B, LEU 142B | PHE 144B |
| Aloe vera compounds | |||
| Mannan | -10 | PRO 100C, GLN 102B, ARG 103B, GLN 102A, PRO 100°, GLU 116B | GLU 116C, ARG 103°, GLU 104C, PRO 100B |
| Folacin | -9.8 | SER 99B, SER 99C GLU 116B, GLN 102A | GLN 102C, GLU 116A |
| Aloesin | -9.8 | ALA 22B, GLY 24B, LYS 65B, ASP 140B | - |
| Beta sitosterol | -9.6 | GLU 116C, LYS 98B | ARG 103B |
| Campesterol | -9.6 | GLU 116B | ARG 103B |
| Lophenol | -9.4 | GLU 116A | ARG 103B |
| Galactomannan | -9.4 | THR 105B, ARG 103B, ARG 103A, GLN 102A, GLN 102B, GLU 104A, GLU 104B | GLU 107B |
| Cholesterol | -9.3 | GLU 116B, LYS 98C | ARG 103B, LYS 98B |
| Quercetin | -9.1 | GLN 102A, PRO 100A, GLU 116C, GLN 102C | |
| Kaempferol | -9.1 | ASN 34A, GLN 125C, THR 7A, LEU 37A | LEU 36A |
Table 2: Molecular Docking Results of Aloe Vera Compounds against TNF Alpha and The Interacting Amino Acids
Folacin and aloesin both demonstrated strong binding with docking scores of -9.8 kcal/mol. Folacin formed hydrogen bonds with SER 99B, SER 99C, GLU 116B, and GLN 102A, while also engaging in hydrophobic interactions with GLN 102C and GLU 116A. Aloesin, interestingly, formed four hydrogen bonds with Alanine (ALA) 22B, Glycine (GLY) 24B, Lysine (LYS) 65B, and Aspartic acid (ASP) 140B, without any notable hydrophobic interactions. β-sitosterol and campesterol both showed binding energies of -9.6 kcal/mol, with β-sitosterol forming hydrogen bonds with GLU 116C and LYS 98B, and campesterol with GLU 116B. Both compounds shared a hydrophobic interaction with ARG 103B. These high-energy interactions suggest strong binding potential between the aloe vera compounds and TNF-α. The stability of the ligand-receptor complex is attributed to hydrogen bonds formed by OH and C=O groups, with the ligand playing a dual role as acceptor and donor[10]. This interaction, coupled with dispersion forces[11], π-π interactions, and hydrophobic interactions, particularly involving polar amino acids, contributes to the overall stability of the complex[12,13].
To contextualize our findings, we compared the binding energies of aloe vera compounds with those of standard anti-inflammatory drugs (Table 3). Notably, all of the top-performing aloe vera compounds exhibited higher binding affinities than the standard anti-inflammatory drugs. This suggests that these compounds, particularly mannan, folacin, and aloesin, may have significant potential as TNF-α inhibitors. This inhibition could play a crucial role in reducing inflammation associated with conditions like rheumatoid arthritis. However, the anti-inflammatory effects of aloe vera likely extend beyond simple TNF-α inhibition. Recent studies have highlighted the importance of crosstalk between Interleukin (IL-9) and TNF-α in modulating inflammatory responses, playing a crucial role in the anti-inflammatory pathway[4]. This interaction involves reciprocal regulation, shared signalling pathways, and cell-specific effects, contributing to the complexity of inflammatoryregulationindifferenttissues. Inthecontext of our study on aloe vera compounds targeting TNF-α, understanding this crosstalk is crucial. The potential inhibition of TNF-α by aloe vera phytochemicals may not only directly reduce inflammation but also indirectly modulate IL-9 signalling, potentially contributing to the overall anti-inflammatory properties of aloe vera observed in various studies. The complex interplay between different inflammatory mediators underscores the potential advantages of multi-target therapeutic approaches, such as those offered by plant- derived compounds. Aloe vera, with its diverse array of bioactive molecules, may be particularly well-suited to modulate these intricate inflammatory networks.
| Code | Compound | Molecular weight (Da) | Log p | HBD | HBA | Violation | Yes/No | Solubility | Log S (mol/l) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mannan | 666.58 | 0.53 | 14 | 21 | 3 | No | Highly soluble | 2.5 |
| 2 | Folacin | 441.4 | 0.04 | 6 | 9 | 2 | No | Soluble | -2.91 |
| 3 | Aloesin | 394.37 | 0.92 | 5 | 9 | 0 | Yes | Very soluble | -1.53 |
| 4 | Beta sitosterol | 414.71 | 5.05 | 1 | 1 | 1 | Yes | Poorly soluble | -9.67 |
| 5 | Campesterol | 400.68 | 4.97 | 1 | 1 | 1 | Yes | Poorly soluble | -9.11 |
| 6 | Lophenol | 400.68 | 5.06 | 1 | 1 | 1 | Yes | Poorly soluble | -9.02 |
| 7 | Galactomannan | 504.44 | 0.13 | 11 | 16 | 3 | No | Highly soluble | 1.38 |
| 8 | Cholesterol | 386.65 | 4.89 | 1 | 1 | 1 | Yes | Poorly soluble | -9.02 |
| 9 | Quercetin | 302.24 | 1.63 | 5 | 7 | 0 | Yes | Soluble | -3.91 |
| 10 | Kaempferol | 286.24 | 1.7 | 4 | 6 | 0 | Yes | Soluble | -3.86 |
Table 3: LIPINSKI Parameters for Dataset from Swissadme
Our investigation also extended to assessing the pharmacokinetics and toxicity properties of molecules exhibiting promising results, positioning them as potential drug candidates. The selection of potential inhibitors or optimal docked ligands was based on their binding energy, a critical factor in determining their efficacy. However, in the context of drug development, the evaluation of Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) properties is essential.
The assessment of physicochemical properties, a key parameter influencing efficacy, safety, and metabolism, was carried out employing appropriate methodologies, emphasizing the significance of these properties in the drug development and discovery processes. In evaluating the physicochemical properties using RO5, which includes criteria such as molecular mass, hydrogen- bond donors, hydrogen-bond acceptors, and logP, the results demonstrated that among the top ten ligands, only 3 ligands fully complied with Lipinski's rule. Lipinski’s screening serves as a crucial filter in the drug design process, determining the suitability of a compound for further development[14]. The polysaccharides mannan and galactomannan, along with the steroids campasterol, β-sitosterol, cholesterol, and lophenol, exhibited a noteworthy affinity for TNF-α. However, their potential use as drugs is hindered by a failure to comply with essential pharmacological parameters and a violation of the Lipinski rule of drug-likeness. In contrast, the chromone aloesin, and the flavonoids quercetin and kampferol, emerged as promising TNF-α inhibitors. These compounds not only demonstrated high affinity but also met all pharmacological parameters, showing potential for effective drug development. Importantly, these compounds adhered to pharmacological rules and displayed lead-like properties, as outlined in Table 3.
This suggests a potential avenue for the development of these phytochemicals into drug molecules specifically targeting the cytokine TNF-α. Table 4 presents the pharmacokinetic and toxicity properties of the three potential inhibitors such as mannan, folacin, and aloesin. The Ames test (Ames_test), assessing mutagenicity, indicates that all 3 ligands are non- mutagenic. Carcinogenicity in rats (carcino_rat) is negative for all ligands, suggesting no carcinogenic potential. None of the ligands are predicted to permeate the Blood-Brain Barrier (BBB non-permeant). Mannan and folacin show hERG type 1 (hERG1) inhibition, indicating a potential risk for cardiac arrhythmia, while aloesin does not inhibit hERG I. All ligands are non-substrates for P-glycoprotein (P-gp), suggesting a low likelihood of causing drug interactions related to P-gp. Folacin and aloesin are predicted to have hepatotoxicity, while mannan is absence. None of the ligands show skin sensitization. Regarding cytochrome P450 inhibition, all ligands exhibit no inhibitory effects on the assessed isoforms (1A2, 2C19, 2C9, 2D6, 3A4). Overall, these results suggest that aloesin demonstrates a more favourable toxicity profile compared to mannan and folacin, making it a promising candidate for further drug development.
| Ligand | Ames_test | Carcino_rat | BBB permeant | hERG I | hERG II | P-gp S | Hepatotoxicity | Skin sensitization | 1A2 | 2C19 | 2C9 | 2D6 | 3A4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mannan | No | Negative | No | No | Yes | Yes | No | No | No | No | No | No | No |
| Folacin | No | Negative | No | No | No | No | Yes | No | No | No | No | No | No |
| Aloesin | No | Negative | No | No | No | No | Yes | No | No | No | No | No | No |
Table 4: Pharmacokinetics and Toxicity Properties of The 3 Potential Inhibitors
In addressing the global burden of rheumatoid arthritis, this study investigates the anti-inflammatory potential of aloe vera phytochemicals targeting TNF-α. Molecular docking studies identified promising compounds, such as aloesin, quercetin, and kampferol, exhibiting high affinity for TNF-α. Despite Lipinski's rule violations for some compounds, aloesin stands out for its superior binding energy and drug like properties. Future directions include in vivo studies, biological assays, compound optimization, exploring combination therapy, and progressing to clinical trials. These findings suggest a potential avenue for developing novel and effective therapeutics for rheumatoid arthritis.
Conflict of interest:
The authors declared no conflict of interests.
References
- Roy DN, editor. Terpenoids against human diseases. CRC Press; 2019.
- Datta S, Singh V, Nag S, Roy DN. Marine-derived cytosine arabinoside (Ara-C) inhibits biofilm formation by inhibiting PEL operon proteins (Pel A and Pel B) of Pseudomonas aeruginosa: An in silico approach. Mol Biotechnol 2024:1-5.
[Crossref] [Google Scholar] [PubMed]
- Babaahmadi M, Tayebi B, Gholipour NM, Kamardi MT, Heidari S, Baharvand H, et al. Rheumatoid arthritis: The old issue, the new therapeutic approach. Stem Cell Res Ther 2023;14(1):268.
[Crossref] [Google Scholar] [PubMed]
- Roy DN, Goswami R. IL-9 signaling pathway: An update. Methods Mol Biol 2017:37-50.
[Crossref] [Google Scholar] [PubMed]
- Nagargoje MV. Anti-inflammatory properties of aloe vera gel.
- Tyagi N, Tyagi A, Rastogi R, Singh B, Nagarajan K. Unlocking radiance: The dynamic duo of flaxseeds and aloe vera in hair mask for bioactive brilliance. Proteins 2023;22:29.
- Pol N, Raut A, Dhale C. Use of anti-inflammatory drugs in aloe vera gel base formulation. Int J Pharm Sci 2023:26;1(12):1.
- Sayyad S, Shelke P, Sanap G. Review of anti-inflammatory herbal drugs and their impact on health. Int J Pharm Sci 2024;2(1):165-79.
- Rauf B, Alyasi S, Zahra N, Ahmad S, Sarwar A, Aziz T, et al. Evaluating the influence of Aloe barbadensis extracts on edema induced changes in C-reactive protein and interleukin-6 in albino rats through in vivo and in silico approaches. Acta Biochim Pol 2023;70(2):425-33.
[Crossref] [Google Scholar] [PubMed]
- Matondo A, Mukeba CT, Muzomwe M, Nsimba BM, Tsalu PV. Unravelling syn-and anti-orientation in the regioselectivity of carbonyl groups of 5-fluorouracil an anticancer drug toward proton donors. Chem Phys Lett 2018;712:196-207.
- Trujillo C, Sánchez‐Sanz G. A study of π–π stacking interactions and aromaticity in polycyclic aromatic hydrocarbon/nucleobase complexes. Chemphyschem 2016;17(3):395-405.
[Crossref] [Google Scholar] [PubMed]
- Kasende OE, Matondo A, Muya JT, Scheiner S. Interactions between temozolomide and guanine and its S and Se‐substituted analogues. Int J Quantum Chem 2017;117(3):157-69.
- Muya JT, Mwanangombo DT, Tsalu PV, Mpiana PT, Tshibangu DS, Chung H. Conceptual DFT study of the chemical reactivity of four natural products with anti-sickling activity. SN Appl Sci 2019;1:1-8.
- Patel AR, Patel HB, Mody SK, Singh RD, Sarvaiya VN, Vaghela SH, et al. Virtual screening in drug discovery. J Vet Pharmacol Ther 2021;20(2):1-9.