- *Corresponding Author:
- Aimei Tang and Yanli Zhu
Department of Pediatrics,
Maternal and Child Health Hospital of Lianshui County,
Huaian 223400,
China
E-mail: 1455336972@qq.com
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “212-216” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Increasing numbers of children are suffering from hypoxia-related visual impairments such as retinopathy of prematurity. This study aimed to investigate whether all trans retinoic acid reverses hypoxia related impairments in retinal vasculature. Oxygen induced retinopathy model was established in mouse and the expression of ciliary neurotrophic factor, vascular endothelial growth factor and hypoxia inducible factor 1 was detected. We found that ciliary neurotrophic factor level decreased and vascular endothelial growth factor and hypoxia inducible factor 1 levels increased in model mice. However, all trans retinoic acid suppressed the expression of retinal vascular endothelial growth factor and hypoxia inducible factor 1 while upregulated retinal ciliary neurotrophic factor expression in model mice. In conclusion, all trans retinoic acid can reverse the effects of hypoxia on retinal neovascularization and attenuate retinal neurodegeneration. All trans retinoic acid is a potential agent for the prevention and therapy of retinopathy of prematurity.
Keywords
Retinoic acid, vascular endothelial growth factor, ciliary neurotrophic factor, hypoxia inducible factor, retinopathy
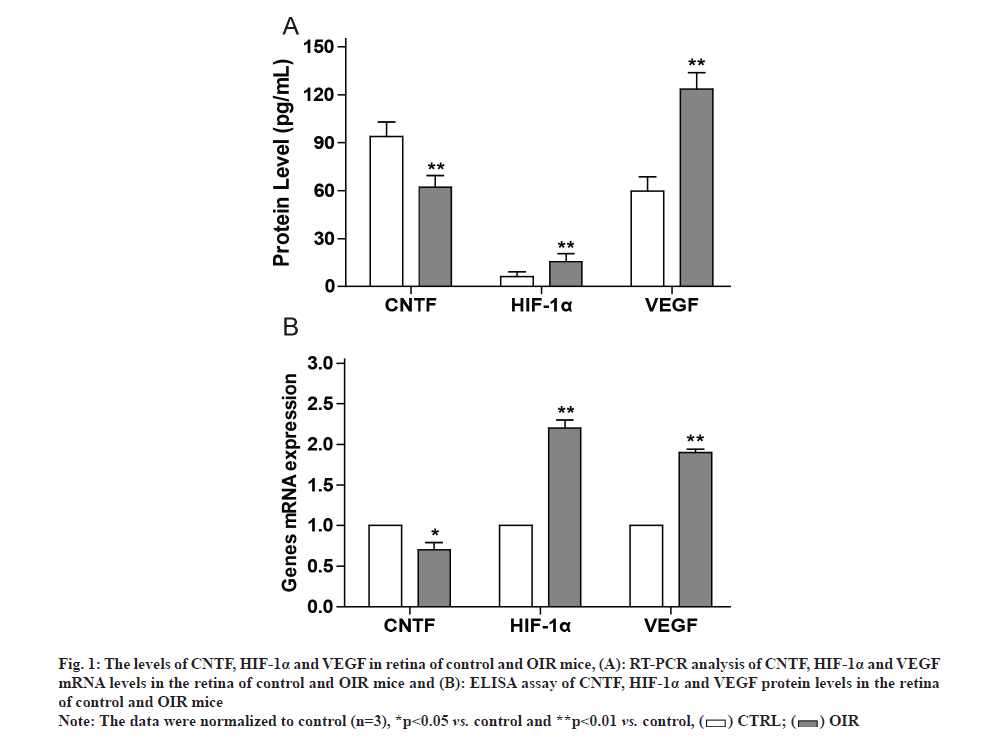
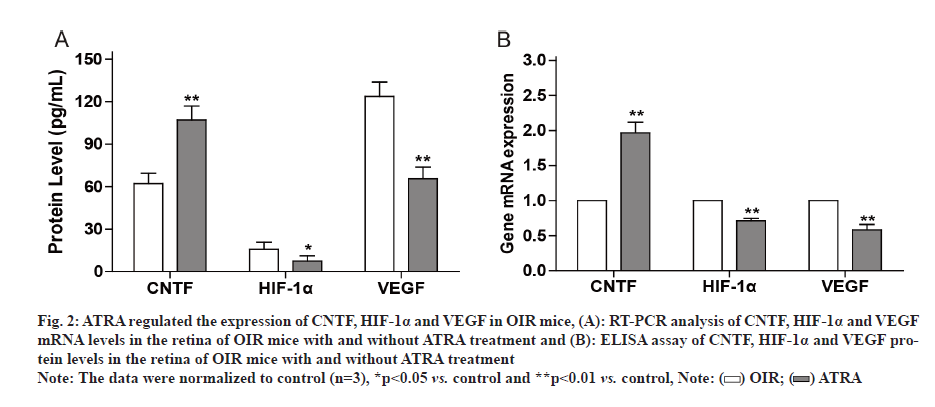
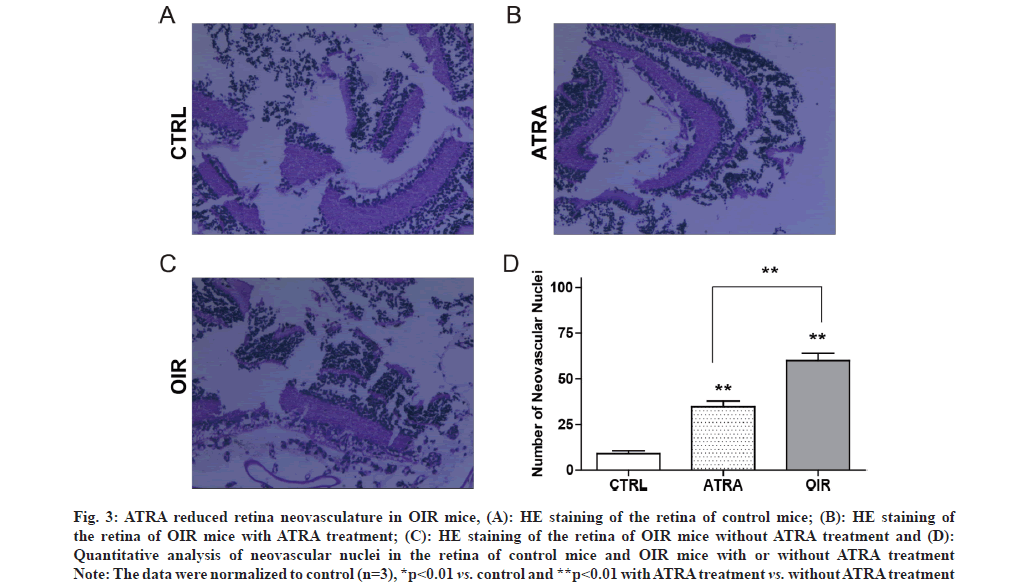
The retina is particularly vulnerable to hypoxia, such as Retinopathy of Prematurity (ROP). One important aspect of ROP is local ischemia, which affects both retinal vasculature and neural retina. ROP is a common cause of blindness and vision impairment in preterm infants worldwide [1-3]. Therefore, it is important to maintain normal vision by protecting against or even counteracting, retinal neovascularization and neurodegeneration. It is known that Hypoxia Inducible Factor (HIF)-1 alpha (α)/Vascular Endothelial Growth Factor (VEGF) axis is implicated in ROP [4]. VEGF plays a role in retinal neovascularization which could promote ROP. HIF-1α regulates gene expression in response to hypoxia and stimulates angiogenesis. Retinal neovascularization has been a focus for current strategies for ROP therapy, but the neuroregeneration of the retina is ignored as potential therapeutic approach. For example, Ciliary Neurotrophic Factor (CNTF) is an important neurotrophic factor involved in retinal degenerative disorders that regulates the development, differentiation and survival of retinal neurons [5]. All Trans Retinoic Acid (ATRA) is an active metabolite of vitamin A and exhibits activity on cell proliferation, differentiation and apoptosis [6,7]. We previously reported that ATRA modulated the expression of Orosomucoid 1-Like 3 (ORMDL3) and CD2-Associated Protein (CD2AP) [8,9]. However, whether ATRA could modulate retinal neovascularization and rejuvenate neurodegeneration is unclear. In present study, we established Oxygen-Induced Retinopathy (OIR) model in the mice and observed that CNTF level decreased while VEGF and HIF-1 levels increased in OIR mice. However, exposure of OIR mice to ATRA inhibited retinal VEGF and HIF-1 expression while upregulated retinal CNTF expression. Animal experiments were approved by Animal Care and Use Committee at Lianshui County People’s Hospital and the efforts were made to minimize suffering. Seventy C57 Black 6 (C57BL/6J) newborn mice were provided by Model Animal Center at Nanjing University. OIR was induced by exposure to 75 %±2 % oxygen in mice from Postnatal D 7 (P7) to Postnatal D 12 (P12). In control group, the mice were exposed to room air. ATRA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in Dimethyl Sulfoxide (DMSO). ATRA was intragastrically administered at 5 mg/kg daily for 6 d (from P13 to P18) in OIR mice, while equal volume of DMSO was intragastrically administered in control mice. C57BL/6J newborn mice were sacrificed at postnatal day (P19) by decapitation. The retina was dissected and Hematoxylin and Eosin (H&E) staining was used to assess retinal vascularization as described previously [10]. Briefly, the stained sections were examined for neovascular tufts that projected from the retina into the vitreous. The mean number of neovascular nuclei per section was calculated. Total Ribonucleic Acid (RNA) was extracted from the retina using Trizol (Invitrogen). Real-time Polymerase Chain Reaction (RT-PCR) was performed using SYBR Master Mix (Roche) on Applied Biosystems 7300 PCR System (Life Technologies). The primers were as follows: CNTF 5’-GGGACCTCTGTAGCCGCTCTATCTG-3’ and 5’-GTTCCAGAAGCGCCATTAACTCCTC-3’; Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) 5’-GGTGAAGGTCGGTGTGAACG-3’ and 5’-TTGGCTCCACCCTTCAAGTG-3’. VEGF 5’-TTCG T C C A A C T T C T G G G C T C T- 3 ’ a n d 5’-CTCCTCTTCCTTCTCTTTCTCCCC-3’. GAPDH was used as an internal control as described previously [11]. Lysates of retinal tissues were prepared and the levels of CNTF, VEGF and HIF-1α in the lysates were detected with Enzyme-Linked Immunosorbent Assay (ELISA) kits (R&D systems) according to the manufacturer’s protocols. All data are expressed as mean±Standard Deviation (SD) and analyzed by oneway analysis of variance followed by Tukey’s test using Statistical Package for the Social Sciences (SPSS) 21.0 software. p<0.05 indicated significant difference. It is known that the pathogenesis of ROP involves retinal vasculature paraplasm and retinal degeneration [12]. RTPCR showed that VEGF and HIF-1α messenger RNA (mRNA) levels were significantly higher while CNTF mRNA level was significantly lower in retinal tissues of OIR mice compared to control mice as shown in fig. 1A. ELISA showed that VEGF and HIF-1α protein levels were significantly higher while CNTF protein level was significantly lower in retinal tissues of OIR mice compared to control mice as shown in fig. 1B. It was reported that retinoic acid inhibited VEGF expression in Schwann cells [13]. Thus we hypothesized that ATRA may inhibit retinal neovasculature. RT-PCR showed that after ATRA treatment, VEGF and HIF- 1α mRNA levels significantly decreased while CNTF mRNA level significantly increased in retinal tissues of OIR mice as shown in fig. 2A. ELISA showed that after ATRA treatment, VEGF and HIF-1α protein levels significantly decreased while CNTF protein level significantly increased in retinal tissues of OIR mice as shown in fig. 2B. Compared to control mice as shown in fig. 3A, HE staining showed extensive retina neovasculature in OIR mice as shown in fig. 3B. However, after ATRA treatment retina neovasculature was reduced in OIR mice as shown in fig. 3C. Quantitative analysis indicated that neovascular nuclei were decreased by nearly 58.33 % in ATRA group (35.0±6.3) compared to OIR group (60.2±5.7) as shown in fig. 3D. These data suggest that ATRA may inhibit retinal neovascularization via decreasing VEGF and HIF-1 expression. ROP is a common cause of vision impairment in preterm infants and current treatments for ROP focus on retinal neovascularization because retinal neovascularization could promote ROP. Therefore, this study aimed to evaluate the effects of ATRA on retinal neovascularization. In this study, we established OIR model in the mice to mimic ROP in human. Based on this model we found that the levels of pro-angiogenic factors VEGF and HIF-1 increased significantly in the retina of OIR mice but decreased significantly in the retina of OIR mice treated with ATRA. Our results are in agreement with previous report that ATRA impaired vasculogenic mimicry of U87 stem-like cells [14]. These data suggest that ATRA may downregulate retinal VEGF and HIF-1 expression to inhibit retinal neovascularization. On the other hand, CNTF is an important neurotrophic factor that inhibits retinal degeneration [15-17]. We found that the levels of CNTF decreased significantly in the retina of OIR mice but increased significantly in the retina of OIR mice treated with ATRA. These results indicate that ATRA may upregulate retinal CNTF expression to promote retinal regeneration. However, Hasler et al. reported that retinoic acid led to decreased CNTF expression [18]. The reason may be due to the difference in the dose and route of administration of ATRA. ATRA may induce the expression of CNTF in the retina to prevent retinal apoptosis [19,20]. In conclusion, ATRA inhibited HIF-1 induced VEGF upregulation and reduced retinal neovascularization in OIR mice. Moreover, ATRA induced the expression of CNTF in the retina. The exposure of OIR mice to ATRA can reverse the effects of hypoxia on retinal neovascularization. ATRA is a promising agent for adjunct therapy of ROP.
Fig. 1: The levels of CNTF, HIF-1α and VEGF in retina of control and OIR mice, (A): RT-PCR analysis of CNTF, HIF-1α and VEGF mRNA levels in the retina of control and OIR mice and (B): ELISA assay of CNTF, HIF-1α and VEGF protein levels in the retina of control and OIR mice
Note: The data were normalized to control (n=3), *p<0.05 vs. control and **p<0.01 vs. control,
Fig. 2: ATRA regulated the expression of CNTF, HIF-1α and VEGF in OIR mice, (A): RT-PCR analysis of CNTF, HIF-1α and VEGF mRNA levels in the retina of OIR mice with and without ATRA treatment and (B): ELISA assay of CNTF, HIF-1α and VEGF protein levels in the retina of OIR mice with and without ATRA treatment
Note: The data were normalized to control (n=3), *p<0.05 vs. control and **p<0.01 vs. control,
Fig. 3: ATRA reduced retina neovasculature in OIR mice, (A): HE staining of the retina of control mice; (B): HE staining of the retina of OIR mice with ATRA treatment; (C): HE staining of the retina of OIR mice without ATRA treatment and (D): Quantitative analysis of neovascular nuclei in the retina of control mice and OIR mice with or without ATRA treatment
Note: The data were normalized to control (n=3), *p<0.01 vs. control and **p<0.01 with ATRA treatment vs. without ATRA treatment
Acknowledgements:
We thank Dr. Wendi Zhou for his help in this work.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382(9902):1445-57.
[Crossref] [Google Scholar] [PubMed]
- Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 2008;84(2):77-82.
[Crossref] [Google Scholar] [PubMed]
- Hussain N, Clive J, Bhandari V. Current incidence of retinopathy of prematurity, 1989–1997. Pediatrics 1999;104(3):e26.
[Crossref] [Google Scholar] [PubMed]
- Li H, Liao C, Weng W, Zhong H, Zhou T. Association of hypoxia-inducible factor-1α (HIF1α) 1772C/T gene polymorphism with susceptibility to renal cell carcinoma/prostate cancer. Biocell 2020;44(2):257-62.
- Rhee KD, Yang XJ. Function and mechanism of CNTF/LIF signaling in retinogenesis. Adv Exp Med Biol 2010;664:647-54.
[Crossref] [Google Scholar] [PubMed]
- Arendt KL, Zhang Y, Jurado S, Malenka RC, Südhof TC, Chen L. Retinoic acid and LTP recruit postsynaptic AMPA receptors using distinct SNARE-dependent mechanisms. Neuron 2015;86(2):442-56.
[Crossref] [Google Scholar] [PubMed]
- Gutierrez-Fernandez A, Soria-Valles C, Osorio FG, Gutierrez-Abril J, Garabaya C, Aguirre A, et al. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J 2015;34(14):1875-88.
[Crossref] [Google Scholar] [PubMed]
- Zhuang LL, Huang BX, Feng J, Zhu LH, Jin R, Qiu LZ, et al. All-trans retinoic acid modulates ORMDL3 expression via transcriptional regulation. PloS one 2013;8(10):e77304.
- Xu HG, Jin R, Gao S, Ren W, Zou L, Liu L, et al. All-trans retinoic acid up-regulates the human CD2AP gene expression through Sp1/Sp3 binding sites. Immunol Res 2015;62(3):273-9.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn 2003;228(4):630-42.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez RA, Polston PM, Wu MJ, Wu J, Sobsey MD. An improved infectivity assay combining cell culture with real-time PCR for rapid quantification of human adenoviruses 41 and semi-quantification of human adenovirus in sewage. Water Res 2013;47(9):3183-91.
[Crossref] [Google Scholar] [PubMed]
- Zin A, Gole GA. Retinopathy of prematurity-Incidence today. Clin Perinatol 2013;40(2):185-200.
[Crossref] [Google Scholar] [PubMed]
- Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015;122(1):200-10.
[Crossref] [Google Scholar] [PubMed]
- Ling GQ, Liu YJ, Ke YQ, Chen L, Jiang XD, Jiang CL, et al. All-trans retinoic acid impairs the vasculogenic mimicry formation ability of U87 stem-like cells through promoting differentiation. Mol Med Rep 2015;12(1):165-72.
- Li Y, Tao W, Luo L, Huang D, Kauper K, Stabila P, et al. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PloS one 2010;5(3):e9495.
[Crossref] [Google Scholar] [PubMed]
- Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res 2012;31(2):136-51.
[Crossref] [Google Scholar] [PubMed]
- He Y, Guo L, Ding J, Lv H, Ma Q, Chen L, et al. Down-regulation of N-methyl-D-aspartate receptor subunits 1 affects neurogenesis of hippocampal neural stem cells. Biocell 2021;45(2):417.
- Hasler PW, Brandi Bloch S, Villumsen J, Fuchs J, Lund-Andersen H, Larsen M. Safety study of 38 503 intravitreal ranibizumab injections performed mainly by physicians in training and nurses in a hospital setting. Acta Ophthalmol 2015;93(2):122-5.
[Crossref] [Google Scholar] [PubMed]
- Li XR, Lv XY, Tan AR, Han JH, Li CY, Bao TH. miR101 aggravates cell apoptosis in cerebral ischemic area by downregulating JAK2. Neuropsychiatr Dis Treat 2021;17:2791-802.
- Zhang Z, Li R, Zhang X, Wei Y, Ma H, Zhu L, et al. Voluntary exercise promotes neurotrophic factor and suppresses apoptosis in hippocampal ischemia. J Integr Neurosci 2019;18(1):65-70.
[Crossref] [Google Scholar] [PubMed]