- *Corresponding Author:
- Hai Bin Luo
Key Laboratory of Tropical Biological Resources of Ministry of Education, and Hainan Engineering Research Centre for Drug Screening and Evaluation, Haikou, 570228,China
E-mail: hbluo@hainanu.edu.cn
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “108-114” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Oral submucous fibrosis is a common precancerous state, which is characterized by abnormal collagen deposition, epithelial atrophy and microvascular disease. There are many factors that may contribute to the development of the pathogenesis, including environmental factors, genetic predisposition, and immune system abnormalities. About 7 %-13 % of oral submucous fibrosis cases can transform into malignant tumors. Therefore, how to establish an animal model with the same pathological mechanism as human oral submucous fibrosis is an urgent problem to be solved. For animal models, researchers have successfully established various animal models of oral submucous fibrosis by transplanting human oral mucosal tissue or inducing the condition with specific drugs. These models provide a powerful tool for studying the pathogenesis and treatment of oral submucous fibrosis. Consequently, this paper summarized the pathogenesis of oral submucous fibrosis and six methods of constructing the oral submucous fibrosis animal models, so as to identify new drug targets for the development of anti-oral submucous fibrosis drugs, lay a theoretical foundation and provide new treatment thinking for clinical work.
Keywords
Oral submucous fibrosis, pathogeny, arecoline, animal models, collagen
Oral Submucous Fibrosis (OSF) was first described by Schwartz in 1952, and the term was first proposed by Jens J. Pindborg and Satyavati M. Sirsat in 1966, which is still in use today[1,2]. OSF is a chronic, progressive and scarred precancerous state, which is characterized by excessive and abnormal collagen deposition in laminae propria layer and submucous layer, leading to tissue fibrosis, glassy degeneration and muscular degeneration[2-6].
OSF was originally restricted in India, but it has not spread to Asian populations in America, England, China and Southeast Asia, which has gradually become a severe health issue in the world[7-11]. According to the statistics of the World Health Organization (WHO), there are over 5 million patients with OSF worldwide[4,11,12]. For the time being, animal model with identical pathogenesis to human OSF has not been built, and how to completely mimic an animal model similar to human OSF is an urgent problem which needs to be solved. Therefore, this paper summarized the etiology, pathogenesis and animal model construction methods of OSF, with an aim to provide new drug targets for the development of anti-OSF drugs, and to provide theoretical foundation and a new treatment thinking for clinical practice.
Etiology of OSF
The etiology of OSF remains unclear, and it is currently considered that it is an oral mucosal disease might be caused by multiple factors. As suggested by epidemiologic studies, betel nut chewing is one of the most important risk factors for inducing OSF[13-15]. Betel nut contains a large amount of arecoline, which plays an important role in the pathogenesis of OSF as mentioned in current study[16-18]. It is demonstrated in some other studies that the risk of OSF in the betel nut chewing population increases by 109-287 folds compared with the non-chewing population, besides, the betel nut chewing frequency is positively correlated with the duration[19,20].
Other risk factors include smoking, eating spicy foods, vitamins B and C, iron deficiency, gene mutations, autoimmunity and human papilloma virus infection[4,20-22]. The morbidity of OSF varies depending on race and region, and it is also closely correlated with factors such as diet, habit and culture[23-25]. As reported in some study, betel nut chewing, smoking, drinking and other habits will increase the risk of OSF[20,26,27].
Pathogenesis of OSF
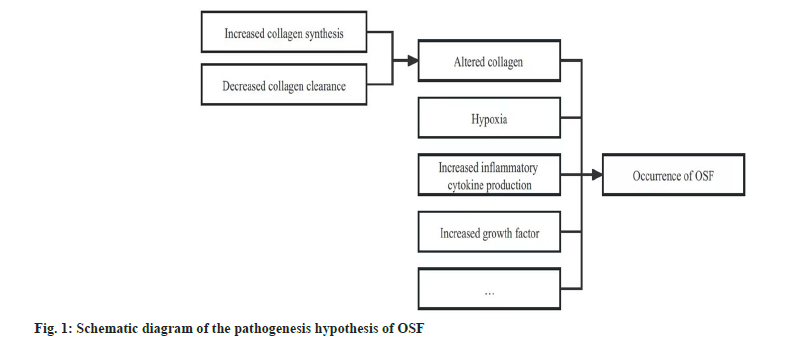
The pathogenesis of OSF is still unclear, which may be related to factors including altered collagen homeostasis, hypoxia, together with increased production of inflammatory cytokines and growth factors[28-32] (fig. 1).
Altered collagen homeostasis:
At the moment, a majority of studies have indicated that, OSF is the consequence of altered collagen homeostasis, namely, the reduced collagen clearance and the increased collagen synthesis[19,30].
Decreased collagen clearance:
Collagen stabilization, defective Extracellular Matrix (ECM) dynamics and suppression of collagen phagocytosis will reduce the collagenase activity, which subsequently decreases collagen degradation, and finally leads to the decreased collagen clearance rate[28,30].
Arecoline promotes cross-linking between collagen peptide chains, thereby endowing collagen the ability to resist collagenase-mediated degradation. Besides, arecoline dose-dependently up-regulates cystatin C in buccal mucosal fibroblasts[30,33-35]. The up-regulation of cystatin C in turn inhibits the cysteine protease, thus decreasing collagen degradation and further stabilizing collagen in OSF[36]. Flavonoids such as tannin and catechin are other important components in betel nut, which exert synergistic effects with alkaloids to decrease collagen degradation by suppressing collagenase and stabilizing collagenous fiber, finally inducing the occurrence of OSF[36,37].
Fibroblast phagocytosis exerts a critical function in regulating ECM remodeling through collagen degradation[30]. However, fibroblasts derived from OSF patients lack the effective collagen phagocytotic function, which may be related to the ECM deposition and eventually fibrosis[13,38].
Transforming Growth Factor-Beta (TGF-β) can activate Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) and plasminogen activator inhibitors genes to suppress collagen degradation, thus stabilizing collagen in ECM[30,34,39,40]. The reduced levels of Matrix Metalloproteinases (MMPs) secreted by fibroblasts (including MMP-2, MMP- 8 and MMP-9), and the elevated level of TIMP-1 breaks the ECM balance in OSF, thus leading to the increased secretion of ECM[41-43]. These factors can lead to the increased production of collagen[28].
Increased collagen synthesis:
Betel nut-induced local mucosal inflammation will recruit activated T cells and macrophages, and increase the levels of cytokines and TGF-β[44,45]. TGF-β can activate the procollagen genes Collagen Alpha (α) 2(I) (COL1A2), COL3A1, COL6A1, COL6A3 and COL7A1 chain to remarkably increase collagen production[30,46,47]. At the same time, it promotes the production of procollagenase and up-regulates the secretion of Lysyl Oxidase (LOX) that initiates collagenous fiber crosslinking[ 48].
The active components in betel nut can induce the massive synthesis of collagen in oral mucosal cells. The alkaloids in betel nut will stimulate fibroblasts to synthesize collagen[30]. The role of arecoline in the pathogenesis of OSF is mainly achieved by activating fibroblasts and increasing collagen production[14,49,50]. Copper further increases collagen production and oral fibroblast crosslinking. Meanwhile, catechin and flavonoid induce the occurrence of fibrosis through accelerating collagen production and cross-linking[30,51].
Further, the addition of slaked lime (calcium hydroxide) into betel nut will not only create a kind of alkaline environment, promote the oxidation of polyphenols, and increase the release of Reactive Oxygen Species (ROS), but it also accelerates the hydrolysis of betel nut into arecoline, which can induce change in fibroblast phenotype, thereby increasing the formation of collagen with altered molecular structure[36,52,53].
Hypoxia:
Hypoxia-Inducible Factor-1 (HIF-1) is a kind of nuclear transcription factor existing in hypoxic cell nuclei, which can bind to Deoxy Ribonucleic Acid (DNA) and activates the expression of numerous hypoxia genes in the hypoxic environment[50,54-57]. In the early stage of OSF, the high expression of HIF-1 induces the transcription of downstream cytokines like TGF-β, promotes fibroblast proliferation and collagen synthesis, suppresses collagen degradation, and ultimately results in OSF[19,34].
Increased production of inflammatory cytokines and growth factors:
When chewing betel nut, the crude fiber will damage the oral mucosa, then induce inflammatory response in epidermal cells and activate the macrophages to secrete cytokines[52,58,59]. TGF-β is the major cytokine participating in OSF progression, which regulates the expression of type I collagen and α-Smooth Muscle Actin (SMA) in myofibroblasts[60,61].
As discovered in a study, in vitro arecoline upregulates the inflammatory cytokines and growth factors such as Interleukin-1 (IL-1), IL-6, IL- 8, TGF-β, tumor necrosis factor-α, fibroblast cytokines, and platelet-derived growth factor, and down-regulates the interferon-Gamma (γ) level to promote collagen synthesis, leading to tissue fibrosis[30,62,63]. Moreover, arecoline can stimulate the biosynthesis of Connective Tissue Growth Factor (CTGF) in oral mucosal fibrosis through the ROS, nuclear factor-kappa B, Jun N-terminal Kinase (JNK) and p38 Mitogen-Activated Protein Kinases (p38 MAPK) pathways in a dose- and timedependent manner[30,64]. Changes in cytokines and growth factors will result in fibroblast proliferation and collagen synthesis near the injury site, thereby causing OSF[28,65].
Preparation of OSF Animal Model
There are numerous methods to prepare OSF animal model, among them, six methods together with their merits and demerits have been elaborated below.
Application of bleomycin in preparing the OSF rat model:
Bleomycin is one of the most extensively applied drugs in the preparation of fibrosis model. Some scholars have injected bleomycin into the buccal mucosa of Sprague-Dawley (SD) rats to prepare the OSF animal model.
Gao et al.[66] selected 20 Specific-Pathogen-Free (SPF) grade adult male SD rats weighing (220±20) g in their experiment. After isoflurane anesthesia, 100 μl of the 1 mg/ml bleomycin dilution was injected into bilateral buccal mucosae, respectively, once a day for 8 d consecutively. The results suggested that, after injection of bleomycin for 8 w, the OSF model was successfully prepared. Zhang et al.[67] prepared bleomycin at 0.1 mg/ml, 0.5 mg/ml, 1 mg/ml and 2 mg/ml, injected 100 μl of bleomycin at the above 4 concentrations into bilateral buccal mucosae of rats every day for 8 d consecutively, and the rat model of OSF was prepared.
This method can successfully mimic the clinical and pathological manifestations of human OSF at the animal level, and has been extensively applied, with the merits of rapid preparation and good reproducibility. However, it is still associated with certain limitations; for instance, due to the different pathological process from betel nut chewing, pathological changes show atypical hyperplasia and pulmonary fibrosis, and repeated injections will cause unwanted inflammation, edema and congestion[68].
Construction of OSF mouse model based on the arecoline drinking water method:
As indicated in some research, arecoline is a key factor inducing OSF, and some scholars have adopted the arecoline drinking water method to construct the OSF mouse model. Wen et al.[69] utilized 27 SPF grade male Balb/c mice and fed them with 500 mg/l arecoline dissolved in water for 12 w. According to their observations, early OSF-like lesions were observed on the buccal mucosa. Wen et al.[70] added a high concentration of arecoline (1000 mg/l) in the drinking water, and early OSF-like changes occurred in lingual mucosa in the 8th w, while OSF-like changes were also observed in oral and buccal mucosae in the 12th w, and transparent changes were seen in the lingual and palatal mucosae in the 20th w, indicating the advanced OSF.
Construction of the OSF mouse model through injection of betel nut extract:
It is suggested by numerous studies in vitro that, Areca Nut Extract (ANE) can increase collagen formation through the trans-differentiation of myofibroblasts that express the intracellular marker protein α-SMA, and regulate MMP activity to further decrease collagen degradation. Moreover, ANE can induce fibrosis in different cell lines via signaling molecules such as TGF-β, CTGF, IL-6 and prostaglandin E2[71,72]. To verify the inhibitory effect of photobiomodulation therapy on mouse OSF, Chiang et al.[72] injected ANE into the buccal mucosa and induced fibrosis within 1 mo. Besides, they observed the OSF-like pathological features. Shekatkar et al.[73] injected 50 μl ANE (50 mg/ml) into the right buccal mucosa in Swiss albino mice at 2 d intervals within 12 w and induced OSF.
This model is stable and rapid, but repeated injections will induce unnecessary inflammation, edema and congestion, and interfere with the common OSF formation pathways. In addition, the pathological process is slightly different from betel nut chewing, and the composition of ANE remains unclear.
Preparation of OSF mouse model through applying betel nut extract:
The OSF mouse model is prepared through applying ANE onto the buccal mucosa. Perera et al.[74] randomly selected 20 adult male albino Balb/c mice with the age of 10-12 w (weight, 28-30 g), applied ANE onto the buccal mucosal surface for twice a day, 6 d a w for 300-600 consecutive d, and successfully prepared the OSF model. Yang et al.[75] applied ANE at four concentrations (0, 0.5 mg/ml, 2 mg/ml, and 8 mg/ml) in buccal mucosa of SD rats for 16 w, and the SD rat model of OSF was successfully constructed under the stimulation of 2 mg/ml and 8 mg/ml ANE. However, the model was not constructed by stimulation of 0 and 0.5 mg/ml ANE, revealing that this method requires a certain concentration of ANE when constructing the OSF model.
Compared with other modeling methods, this method can mimic the human OSF pathological process, but it is time-consuming, almost the whole life cycle of mice is needed to induce OSF, and the ANE composition remains unclear. Consequently, numerous confounding factors (especially aging) may affect the pathological process of OSF in the model.
Construction of the OSF mouse model through drip of betel nut extract:
The OSF mouse model is prepared through intravenous drip of ANE in the entire oral cavity. Shekatkar et al.[73] given drip of 50 μl ANE (50 mg/ ml) into the whole oral cavity in the Swiss albino mice every day, mice were not allowed to drink water within 3 h after administration, and the OSF model was constructed after 12 w. Meanwhile, as revealed by the study by Shekatkar et al. the method of oral drip of ANE was superior to oral local injection of ANE. This method is easy in operation, brings fewer discomforts to the mice, and mimics the natural development of the disease, but the ANE composition remains to be further determined.
Construction of the OSF rabbit model through injection of phenol solution:
The rabbit OSF model is constructed through injection of phenol solution into the rabbit buccal mucosa. Lin et al.[76] injected 4 % phenol solution in the buccal mucosa twice a week, and they successfully induced the OSF-like lesions in rabbits 4 w later. Lin et al. used the model to determine the relation between OSF pathological changes and endogenous collagenase activity, and whether exogenous collagenase treatment improved the food intake function. This model is rarely used, and its merits and demerits remain unclear.
Conclusion
OSF is prevalent in Asian countries and has spread in North America and Europe. At present, the etiology of OSF may be related to factors like betel nut chewing, smoking, eating spicy foods, vitamin B, vitamin C and iron deficiency, gene mutations, autoimmunity and human papillomavirus infection. The pathogenesis of OSF is complex and still not fully understood. The pathogenesis of OSF may be associated with factors such as altered collagen homeostasis, hypoxia, together with increased inflammatory cytokine and growth factor production. For the time being, there are mainly six methods for constructing the OSF animal models, which have their own advantages and disadvantages.
The premise of constructing an animal model with identical pathogenic mechanism to human OSF is to determine the etiology and pathogenesis of OSF. Consequently, more studies are needed to further investigate the etiology and pathogenesis of OSF, so as to provide theoretical foundation and new treatment thinking for the prevention and treatment of OSF.
Acknowledgements:
This study was supported by the National Natural Science Foundation of China (No. 82360190), Fundamental Research Funds for Hainan University (No. KYQD (ZR)-21031 and XTCX2022JKA01), Science Foundation of Hainan Province (No. KJRC2023B10) and Hainan Provincial Natural Science Foundation of China (No. 822RC828 and 821MS027).
Author contribution:
Wen Luo, Jie Mei and Kaiyue Zheng contributed equally to this work and shared the first authorship. Xi Xie and Hai Bin Luo are the corresponding authors.
Conflict of interests:
The authors declared no conflict of interest.
References
- Sharma SR, Chavan S, Karjodkar FR, Sansare K, Bharathi S, Singh S. Correlation of clinical features in oral submucous fibrosis: A 9-year retrospective study. Ethiop J Health Sci 2022;32(1):137-44.
- Gupta S, Jawanda MK. Oral submucous fibrosis: An overview of a challenging entity. Indian J Dermatol Venereol Leprol 2021;87(6):768-77.
- Kavitha L, Ranganathan K, Shyam S, Fathima JHS, Umesh W, Warnakulasuriya S. Immunohistochemical biomarkers in oral submucous fibrosis: A scoping review. J Oral Pathol Med 2022;51(7):594-602.
- Peng H, Jiang X, Cui L, Zhu Y, Ye Z, Zhang Z. Mechanistic investigation of curcuma protection against oral submucous fibrosis. Evid Based Complement Alternat Med 2022:1-8.
- Gondivkar SM, Sarode SC, Gadbail AR, Gondivkar RS, Chole R, Sarode GS. Bibliometric analysis of 100 most cited articles on oral submucous fibrosis. J Oral Pathol Med 2018;47(8):781-7.
- Chen PY, Chao SC, Hsieh PL, Liao YW, Chu PM, Harn HJ, et al. Butylidenephthalide abrogates the snail-induced cancer stemness in oral carcinomas. Int J Mol Sci 2022;23(11):6157.
- Chen J, Li W, Liu B, Xie X. Low LINC02147 expression promotes the malignant progression of oral submucous fibrosis. BMC Oral Health 2022;22(1):316.
- Wang FF, Wang T, Li F. Research progress in clinical treatment of oral submucous fibrosis. Chin Gen Med 2022;20(7):1203-6.
- Prabhu RV, Prabhu V, Chatra L, Shenai P, Suvarna N, Dandekeri S. Areca nut and its role in oral submucous fibrosis. J Clin Exp Dent 2014;6(5):e569-75.
- Tilakaratne WM, Ekanayaka RP, Warnakulasuriya S. Oral submucous fibrosis: A historical perspective and a review on etiology and pathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122(2):178-91.
- Muller S, Tilakaratne WM. Update from the 5th edition of the World Health Organization classification of head and neck tumors: Tumours of the oral cavity and mobile tongue. Head Neck Pathol 2022;16(1):54-62.
- Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW. Oral submucous fibrosis: A contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol Head Neck Surg 2020;49(1):3.
- Shen YW, Shih YH, Fuh LJ, Shieh TM. Oral submucous fibrosis: A review on biomarkers, pathogenic mechanisms, and treatments. Int J Mol Sci 2020;21(19):7231.
- Cai X, Huang J. Clinicopathological factors associated with progression of oral submucous fibrosis: A population-based retrospective study. Oral Oncol 2022;130:105949.
- Chhabra AK, Sune R, Reche A. Oral submucous fibrosis: A review of the current concepts in management. Cureus 2023;15(10).
- Xu H, Lyu FY, Song JY, Xu YM, Jiang EH, Shang ZJ, et al. Research achievements of oral submucous fibrosis: Progress and prospect. Biomed Res Int 2021;2021:6631856.
- Yuwanati M, Ramadoss R, Kudo Y, Ramani P, Senthil Murugan M. Prevalence of oral submucous fibrosis among areca nut chewers: A systematic review and meta-analysis. Oral Dis 2023;29(5):1920-6.
- Wang W, Xiong H, Hu Z, Zhao R, Hu Y, Chen W, et al. Experimental study on TGF-β1-mediated CD147 expression in oral submucous fibrosis. Oral Dis 2018;24(6):993-1000.
- Peng Q, Li H, Chen J, Wang Y, Tang Z. Oral submucous fibrosis in Asian countries. J Oral Pathol Med 2020;49(4):294-304.
- Cirillo N, Duong PH, Er WT, Do CT, de Silva ME, Dong Y, et al. Are there betel quid mixtures less harmful than others? A scoping review of the association between different betel quid ingredients and the risk of oral submucous fibrosis. Biomolecules 2022;12(5):664.
- Memon AB, Rahman AA, Channar KA, Zafar MS, Kumar N. Evaluating the oral-health-related quality of life of oral submucous fibrosis patients before and after treatment using the OHIP-14 tool. Int J Environ Res Public Health 2022;19(3):1821. Hernandez BY, Zhu X, Goodman MT, Gatewood R, Mendiola P, Quinata K. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PloS One 2017;12(2):e0172196.
- Singh AG, Roy S, Oza S, Singhavi H, Chatterjee K, Chaturvedi P. A contemporary narrative review to guide molecular epidemiology of oral submucous fibrosis. Int J Mol Epidemiol Genet 2021;12(4):61-70.
- Tang ZG, Cheng YX. Research progress in clinical diagnosis and treatment of oral submucous fibrosis. Oral Med Res 2022;38(8):705-9.
- Chattopadhyay A, Ray JG. Molecular pathology of malignant transformation of oral submucous fibrosis. J Environ Pathol Toxicol Oncol 2016;35(3):193-205.
- Aparnadevi P, Nirmal RM, Veeravarmal V, Nandini DB, Kalyani C, Singh DN, et al. Cyclooxygenase-2 (COX-2) expression in oral submucous fibrosis and oral squamous cell carcinoma: An immunohistochemical study. J Pharm Bioallied Sci 2022;14:S769-s773.
- Anand R, Dhingra C, Prasad S, Menon I. Betel nut chewing and its deleterious effects on oral cavity. J Cancer Res Ther 2014;10(3):499-505.
- Shih YH, Wang TH, Shieh TM, Tseng YH. Oral submucous fibrosis: A review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci 2019;20(12):2940.
- Jian XC, Gao X. Etiology, pathogenesis, diagnosis and treatment of oral submucous fibrosis. Prev Treat Oral Dis 2021;29(4):217-25.
- Arakeri G, Rai KK, Hunasgi S, Merkx MA, Gao S, Brennan PA. Oral submucous fibrosis: An update on current theories of pathogenesis. J Oral Pathol Med 2017;46(6):406-12.
- Aziz SR. Oral submucous fibrosis: Case report and review of diagnosis and treatment. J Oral Maxillofac Surg 2008;66(11):2386-9.
- Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol 1966;22(6):764-79.
- Li J, Yao M, Zhu X, Li Q, He J, Chen L, et al. YAP-induced endothelial-mesenchymal transition in oral submucous fibrosis. J Dent Res 2019;98(8):920-9.
- Hsieh YP, Wu KJ, Chen HM, Deng YT. Arecoline activates latent transforming growth factor β1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallate. J Formos Med Assoc 2018;117(6):527-34.
- Tsai CH, Yang SF, Chen YJ, Chu SC, Hsieh YS, Chang YC. Regulation of interleukin-6 expression by arecoline in human buccal mucosal fibroblasts is related to intracellular glutathione levels. Oral Dis 2004;10(6):360-4.
- Angadi PV, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis: Revisited. Oral Maxillofac Surg 2011;15(1):1-9.
- Arakeri G, Brennan PA. Oral submucous fibrosis: An overview of the aetiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg 2013;51(7):587-93.
- Lin CY, Hsieh PL, Liao YW, Peng CY, Yu CC, Lu MY. Arctigenin reduces myofibroblast activities in oral submucous fibrosis by LINC00974 inhibition. Int J Mol Sci 2019;20(6):1328.
- Kumari P, Debta P, Dixit A. Oral potentially malignant disorders: Etiology, pathogenesis, and transformation into oral cancer. Front Pharmacol 2022;13:825266.
- Kamath VV, Krishnamurthy S, Satelur KP, Rajkumar K. Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis: An immunohistochemical observation. Indian J Med Paediatr Oncol 2015;36(2):111-6.
- Saleem Z, Shaikh AH, Zaman U, Ahmed S, Siddiqui Z, Majeed MM, et al. Analytical study of salivary Mmp-12 expression in oral submucous fibrosis. J Ayub Med Coll Abbottabad 2022;34(2):247-50. James A, Jayan L, Ramadoss R, Arunachalam P. Leaving no stone unturned: Role of profibrotic genes in oral submucous fibrosis-A systematic review. J Oral Maxillofac Pathol 2022;26(2):228-35.
- Kazmi A, Abbas Z, Saleem Z, Haider S, Farooqui WA, Ahmed S. Relation of salivary MMP-8 with oral submucous fibrosis and oral squamous cell carcinoma: A cross sectional analytical study. BMJ Open 2022;12(12):e060738.
- Chen PN, Lin CW, Yang SF, Chang YC. Oral submucous fibrosis stimulates invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by activating MMP-2 and IGF-IR. J Cell Mol Med 2021;25(20):9814-25.
- Rai A, Ahmad T, Parveen S, Parveen S, Faizan MI, Ali S. Expression of transforming growth factor beta in oral submucous fibrosis. J Oral Biol Craniofac Res 2020;10(2):166-70.
- Ray JG, Chatterjee R, Chaudhuri K. Oral submucous fibrosis: A global challenge. Rising incidence, risk factors, management, and research priorities. Periodontol 2000 2019;80(1):200-12.
- Islam S, Muthumala M, Matsuoka H, Uehara O, Kuramitsu Y, Chiba I, et al. How each component of betel quid is involved in oral carcinogenesis: Mutual interactions and synergistic effects with other carcinogens-a review article. Curr Oncol Rep 2019;21(6):53.
- Zhang DH, Zhou XH. Advances in research on the pathogenesis of oral submucous fibrous canceration. Chin J Oral Maxillofac Surg 2020;18(1):71-6.
- Wang YK, Liu CM, Lin T, Fang CY, Yu CC, Yu CH. Inhibition of HIF1A-AS1 impedes the arecoline-induced migration activity of human oral mucosal fibroblasts. J Formos Med Assoc 2020;119(4):879-83.
- Chen X, He Y, Deng Y. Chemical composition, pharmacological, and toxicological effects of betel nut. Evid Based Complement Alternat Med 2021;2021:1-7.
- He FQ, Wang HF, Xu CJ. Trace elements and oral submucous fibrosis. J Clin Stomatol 2019;35(2):124-7.
- Zhang P, Chua NQ, Dang S, Davis A, Chong KW, Prime SS, et al. Molecular mechanisms of malignant transformation of oral submucous fibrosis by different betel quid constituents-does fibroblast senescence play a role? Int J Mol Sci 2022;23(3):1637.
- Qin X, Ning Y, Zhou L, Zhu Y. Oral submucous fibrosis: Etiological mechanism, malignant transformation, therapeutic approaches and targets. Int J Mol Sci 2023;24(5):4992.
- Zhu JY, Lu R. Advances in research on the malignant transformation mechanism of oral submucous fibrosis. J Oral Sci Res 2021;37(10):875-8.
- Cheng RH, Wang YP, Chang JY, Pan YH, Chang MC, Jeng JH. Genetic susceptibility and protein expression of extracellular matrix turnover-related genes in oral submucous fibrosis. Int J Mol Sci 2020;21(21):8104.
- Prasad S, Gupta SC, Tyagi AK. Reactive Oxygen Species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett 2017;387:95-105.
- Sharma M, Shetty SS, Radhakrishnan R. Oral Submucous fibrosis as an overhealing wound: Implications in malignant transformation. Recent Pat Anticancer Drug Discov 2018;13(3):272-91.
- Guo ZX, Zhang Z, Yan JF, Xu HQ, Wang SY, Ye T, et al. A biomaterial-based therapy using a sodium hyaluronate/bioglass composite hydrogel for the treatment of oral submucous fibrosis. Acta Biomater 2022;157:639-54.
- Lee PH, Chu PM, Hsieh PL, Yang HW, Chueh PJ, Huang YF, et al. Glabridin inhibits the activation of myofibroblasts in human fibrotic buccal mucosal fibroblasts through TGF-β/smad signaling. Environ Toxicol 2018;33(2):248-55.
- Hsieh PL, Yu CC. Oral fibrosis and oral cancer: From molecular targets to therapeutics. Int J Mol Sci 2022;23(11):6110.
- Monteiro R, Hallikeri K, Sudhakaran A. PTEN and α-SMA expression and diagnostic role in oral submucous fibrosis and oral squamous cell carcinoma with concomitant oral submucous fibrosis. J Oral Maxillofac Res 2021;12(1):e3.
- Wang L, Tang Z. Immunopathogenesis of oral submucous fibrosis by chewing the areca nut. J Leukoc Biol 2022;111(2):469-76.
- Wang L, Gu L, Tang Z. Cytokines secreted by arecoline activate fibroblasts that affect the balance of TH17 and Treg. J Oral Pathol Med 2020;49(2):156-63.
- Zhou MX, Guo YC, Li K, Tian X. Advances in research on betel nut active components and pharmacological and toxicological effects. Chin Patent Med 2022;44(3):878-83.
- Lee YH, Liao YW, Lu MY, Hsieh PL, Yu CC. LINC00084/miR-204/ZEB1 axis mediates myofibroblastic differentiation activity in fibrotic buccal mucosa fibroblasts: Therapeutic target for oral submucous fibrosis. J Pers Med 2021;11(8):707.
- Gao ZR, Luo Y, Su K, Pan JC, Xie XY, Gao YJ. Role of halofuginone in resisting fibrosis in SD rat model of oral submucous fibrosis. Chin J Pract Stomatol 2018;11(6):343-6.
- Zhang SS, Gong ZJ, Xiong W, Wang X, Min Q, Luo CD, et al. A rat model of oral submucous fibrosis induced by bleomycin. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122(2):216-23.
- Yang B, Fu MF, Tang ZG. Arecoline and mechanical stimulation applied in constructing the rat model of oral submucous fibrosis. West Chin J Stomatol 2019;37(3):260-4.
- Wen QT, Wang T, Yu DH, Wang ZR, Qing HY, Sun Y. Arecoline drinking water adopted to preliminarily construct the Balb/c mouse model of oral submucous fibrosis. J Guangxi Med Univ 2015;32(6):876-8.
- Wen QT, Wang T, Yu DH, Wang ZR, Sun Y, Liang CW. Development of a mouse model of arecoline-induced oral mucosal fibrosis. Asian Pac J Trop Med 2017;10(12):1177-84.
- Chiang MH, Chen PH, Chen YK, Chen CH, Ho ML, Wang YH. Characterization of a novel dermal fibrosis model induced by areca nut extract that mimics oral submucous fibrosis. PLoS One 2016;11(11):e0166454.
- Chiang MH, Lee KT, Chen CH, Chen KK, Wang YH. Photobiomodulation therapy inhibits oral submucous fibrosis in mice. Oral Dis 2020;26(7):1474-82. Shekatkar M, Kheur S, Sanap A, Undale V, Kharat A, Bhalchim V, et al. A novel approach to develop an animal model for oral submucous fibrosis. Med Oncol 2022;39(11):162.
- Perera MWS, Gunasinghe D, Perera PA, Ranasinghe A, Amaratunga P, Warnakulasuriya S, et al. Development of an in vivo mouse model to study oral submucous fibrosis. J Oral Pathol Med 2007;36(5):273-80.
- Yang B, Fu MF, Tang ZG. Arecoline and mechanical stimulation applied in constructing the rat model of oral submucous fibrosis. West China J Stomatol 2019;37(3):260-4.
- Lin HJ, Lin JC. Treatment of oral submucous fibrosis by collagenase: Effects on oral opening and eating function. Oral Dis 2007;13(4):407-13.