- *Corresponding Author:
- I S Suchiang
Department of Pharmacy, Regional Institute of Paramedical and Nursing Sciences, Aizawl, Mizoram 796017
E-mail: ianstewartsuchiang@gmail.com
| Date of Received | 10 September 2021 |
| Date of Revision | 09 Mar 2024 |
| Date of Acceptance | 08 May 2024 |
| Indian J Pharm Sci 2024;86(3):832-838 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study investigated the phytochemical, total phenolic, flavonoid content and in vitro antioxidant of the methanolic extract of the leaf of Ipomoea hederifolia Linn. In vitro antioxidant activity was determined using 2,2-diphenyl-1-picrylhydrazyl, hydroxyl radical, reducing power and phosphomolybdenum method. The total phenolic and total flavonoid contents were also determined using Folin Ciocalteau’s and aluminium chloride method respectively. The preliminary phytochemical screening showed the presence of alkaloids, glycosides, steroids, triterpenoids, flavonoids, phenols and tannins. Results indicated that the total phenolic and flavonoid content were found to be 101.28±0.9238 mg of gallic acid equivalents/g and 14.91±0.672 quercetin equivalent/g respectively. The half-maximal inhibitory concentration values of the extract based on the 2,2-diphenyl-1-picrylhydrazyl was 20.33 µg/ml as compared to standard (ascorbic acid) which is 18.30µg/ml while the half-maximal inhibitory concentration values of the methanolic extract of Ipomoea hederifolia and standard (ascorbic acid) for hydroxyl scavenging activity were found to be 104.9 µg/ml and 16.88 µg/ml respectively. The reducing power of methanolic extract and standard (ascorbic acid) at 25 µg/ml were found to be 0.183±0.002 and 0.396±0.001 respectively and the total antioxidant capacity of methanolic extract and standard (ascorbic acid) at 100 µg/ml and were found to be 0.094±0.0002 and 0.368±0.002 respectively.
Keywords
Ipomoea hederifolia, antioxidant, 2,2-diphenyl-1-picrylhydrazyl, free radicals, hydroxyl, phenolics, flavonoids
Antioxidants are compounds that have the ability to slow down other molecules oxidation process by stopping oxidation chain reactions that are started or continued by free radicals. Thus, the body's exposure to oxidative damage is reduced [1]. Antioxidants work by neutralizing free radicals, which are defined as individual or groupings of atoms with one unpaired electron [2]. Antioxidants can be obtained from synthetic or natural sources. Antioxidants, which are abundant in certain plantbased foods, function to stop oxidation or chemical reactions that produce free radicals and subsequent chain reactions that damage an organism's cells. There are many different kinds of antioxidants found in plants, such as flavonoids, flavones, polyphenols, carotenoids and phytoestrogens. Antioxidants are primarily responsible for scavenging free radicals[3].
Originating in the Americas, Ipomoea hederifolia Linn is an annual herbaceous vine belonging to the family Convolvulaceae. Scarlet morning glory, scarlet creeper, star ipomoea and ivy-leaved morning glory are some of the names that it goes by[4]. The roots are traditionally used for medicinal purposes in Mizoram[5]. For the purpose of treating stomach aches, these roots are scraped and eaten raw. A modified stem, the tuber is also used as a cathartic to aid in the passage of stools and as a herbal remedy for intestinal parasites. Humans value Ipomoea plants because they are rich in alkaloids, which are primarily used medicinally and psychoactively[6].
The survey of literature on this plant results in a very little information in the field of pharmacological activities. Pharmacologically, the stem part of the plant have been reported to possess antioxidant property and phytochemically different bioactive compounds have been reported from the stem of the plant such as alkaloids, glycosides, tannins, flavonoids, steroids and phenols[7]. Isolated compounds have been isolated from the aerial part of the plant such as four glycosidic acids named hederifolic acids A-D[4], and pyrrolizidine alkaloid varieties known as Ipangulines have also been identified[8]. The objective of the study was aimed to perform the phytochemical screening, quantification of flavonoids and phenolic content along with screening of the antioxidant activity of the leaf.
Materials and Methods
Collection and authentication:
Fresh leaves of Ipomoea hederifolia was collected in the month of November and December 2020 from Regional Institute of Paramedical and Nursing Sciences (RIPANS) campus and Zemabawk local area, Aizawl, Mizoram, India. The leaves of the plant were used for the study. The leaves were washed thoroughly with water and shade dried. The herbarium of this plant was prepared and was sent to Botanical Survey of India, Shillong for authentication. The plant was identified and authenticated as Ipomoea hederifolia L. by Dr. N. Odyou, Scientist-E, Botanical Survey of India, Eastern Regional Centre, Shillong. The identification number was BSI/ERC/Tech/2020/1320.
Extraction of crude drug materials:
The collected fresh leaves of the plant were cleaned, by handpicking the foreign materials, washed and dried under shade for about 2 w. The air-dried leaves were crushed to reduce the leaves size for convenience of extraction. The coarse powdered leaves (650 gm) was extracted successively by using soxhlet apparatus with petroleum ether, chloroform and methanol as solvents respectively for about 50 h or until the solvent in the siphon tube became colorless. Then the solvents were recovered by distillation method using rotary evaporator to obtain the concentrated extracts. All of the three extracts were stored in refrigerator and the methanolic extract was used for evaluation of in vitro antioxidant activity.
Chemicals:
Ascorbic acid (Merck), aluminum chloride (Merck), sodium nitrite (LobaChemie Pvt. Ltd.), gallic acid (LobaChemie Pvt. Ltd.), quercetin (LobaChemie Pvt. Ltd.), sodium hydroxide (Merck), Ethylenediaminetetraacetic Acid (EDTA) (Merck), potassium ferricyanide (Loba Chemie Pvt. Ltd.), trichloroacetic acid (Loba Chemie Pvt. Ltd.), folin-Ciocalteu (Merck), 2,2-diphenyl-1- picrylhydrazyl (DPPH) (HiMedia).
Preliminary phytochemical screening:
Phytochemical examinations were carried out for the methanolic extract. The methanolic extract of the leaf of Ipomoea hederifolia was screened for phytochemicals such as alkaloids, glycosides, phenol, tannins, flavonoids, steroids, triterpenoids and saponins [9-11].
Determination of total phenolic content::
The total phenolic content of methanolic extract of Ipomoea hederifolia was determined by Folin- Ciocalteau’s method. To 1.0 ml of the sample, 5.0 ml of Folin Ciocalteau reagent was added. After 3 min, 4.0 ml of 0.7 M Na2CO3 solution was added to the above mixture and the tubes were kept at room temperature for 1 h, after which its absorbance of the resulting blue colour was read at 765 nm against a blank reagent using ultraviolet spectrophotometer. Gallic acid was used as a standard. The total phenolic content was determined from the linear equation obtained from the standard curve of gallic acid[12].
Determination of total flavonoid content:
Total flavonoid content of the methanolic extract was determined by aluminium chloride method. 1 ml of sample was mixed with 2 ml of methanol and 3 ml of 5 % sodium nitrite. After 5 min, 0.3 ml of 10 % aluminium chloride was added. After 5 min, 2 ml of 1 M sodium hydroxide and the total volume was made upto 10 ml with methanol. After 1 h, the absorbance of the mixture was measured at 510 nm against the blank reagent using spectrophotometer. Quercetin was used as standard. The flavonoid content was expressed as mg of quercetin equivalence per gram of extract[13].
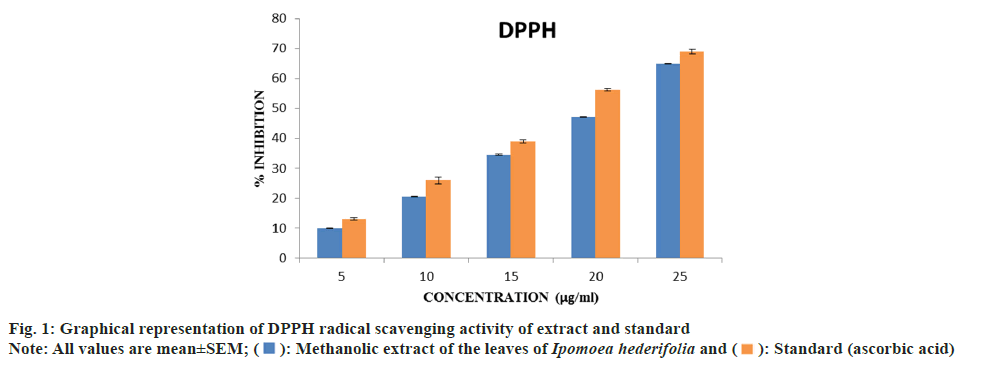
Determination of DPPH radical scavenging activity:
The free radical scavenging activity of the methanolic extract of Ipomoea hederifolia was determined based on the scavenging activity of the stable DPPH free radical. 3 ml of methanolic extract at various concentrations (5, 10, 15, 20 and 25 μg/ml) were taken in a test tubes and 0.5 ml of 0.1 mM DPPH solution was added to each. It was incubated in the dark for 30 min at 37°. The absorbance was measured at 517 nm[14]. The halfmaximal Inhibitory Concentration (IC50) value was calculated. The percentage of DPPH scavenging effect was estimated by using equation
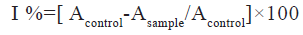
Where, Acontrol is absorbance of control and Asample is absorbance of sample.
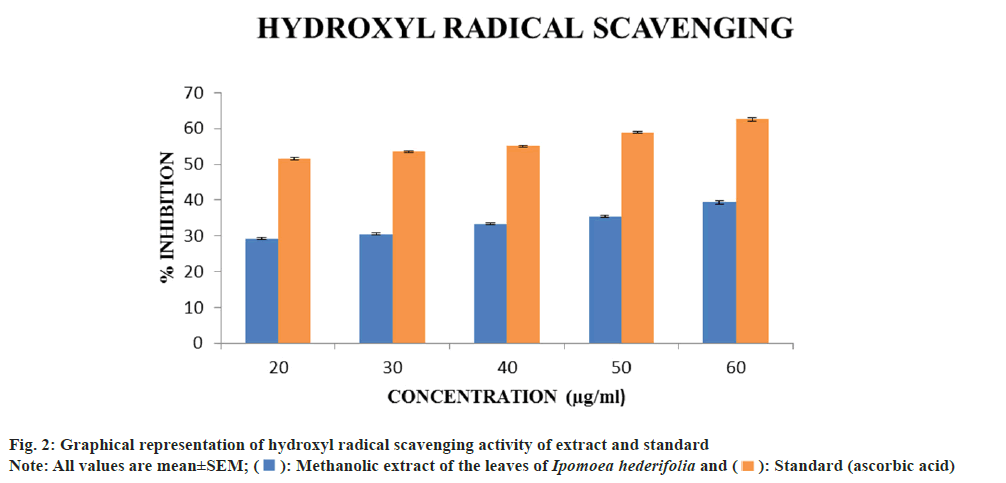
Determination of hydroxyl radical scavenging activity:
1 ml of different concentrations (20, 30, 40, 50 and 60 μg/ml) of extracts were mixed with 1.0 ml of iron EDTA solution (0.13 % ferrous ammonium sulphate, 0.26 % EDTA), 0.5 ml of 0.018 % EDTA, 1.0 ml of Dimethyl Sulfoxide (DMSO) (0.85 % in 0.1 mol/l phosphate buffer pH 7.4) and 0.5 ml of 0.22 % ascorbic acid. The tubes were tapped tightly and heated in water bath at 80°-90° for 15 min, the reaction was terminated by adding 1.0 ml of ice cold Trichloroacetic Acid (TCA) (17.5 %). To the above reaction mixture 3.0 ml of Non- Alcoholic Steatohepatitis (NASH) reagent (75.0 g of ammonium acetate, 3.0 ml of glacial acetic acid and 2.0 ml of acetyl acetone were mixed and distilled water was added up to the volume of 1 l) was added and incubated at room temperature for 15 min for colour development. The intensity of the yellow colour was formed and measured at 412 nm against a reagent blank. Ascorbic acid was used as a standard. The percentage inhibition was determined by comparing test with standard[14]. The percentage inhibition was calculated by the following formula,

Where, Acontrol is absorbance of control and Asample is absorbance of sample.
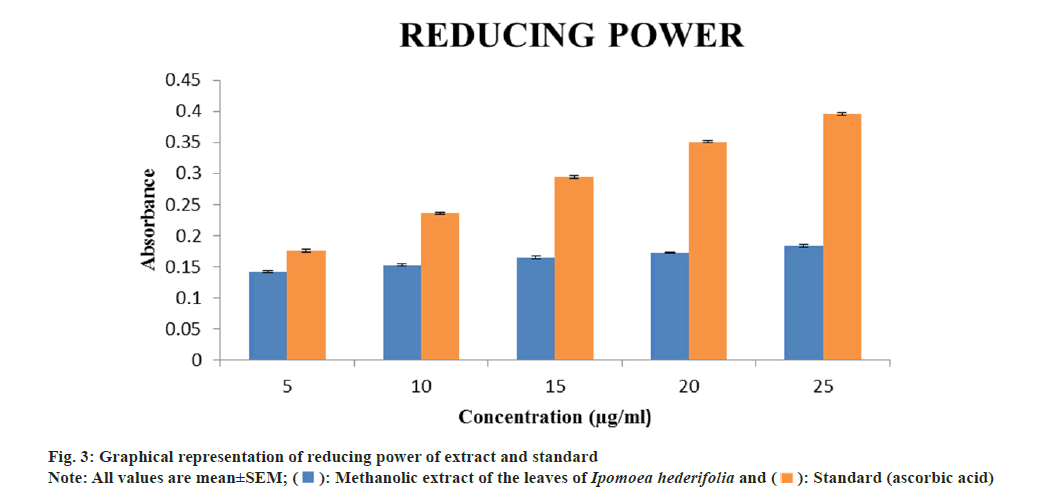
Determination of reducing power assay:
The reducing power of methanolic extract of Ipomoea hederifolia was determined accordingly. The extract was diluted at various concentrations (5, 10, 15, 20 and 25 μg/ml). 1 ml of each dilution was mixed with 2.5 ml of phosphate buffer (pH 6.6) and 2.5 ml of 1 % potassium ferricyanide. The mixture was incubated at 50° for 30 min. After cooling, the reaction was terminated with 2.5 ml of 10 % TCA was added and centrifuge for 10 min at 3000 rpm. 2.5 ml of the supernatant was dilute with 2.5 ml methanol, to it 0.5 ml of freshly prepared 0.1 % ferric chloride solution was added and mixed. The absorbance of the mixture was measured at 700 nm. Ascorbic acid was used as a standard. Increase in absorbance indicates increase in the reducing power[15].
Determination of Total Antioxidant Capacity (TAC) by phosphomolybdenum method:
TAC of the methanolic extract of Ipomoea hederifolia was performed by phosphomolybdenum method. 0.1 ml of extract (20, 40, 60, 80 and 100 μg/ml) was mixed with 1 ml of mixture reagent solution (0.6 M sulphuric acid, 30 mm sodium phosphate and 4 mm ammonium molybdate). The sample tubes were sealed and incubated in a boiling water bath at 95° for 90 min. After incubation the samples were cooled at room temperature. The absorbance of the sample solutions were read at 695 nm against a blank. A blank contain 1.0 ml of reagent and mixed with solvent and incubated under same conditions[16]. Ascorbic acid was used as a standard.
Statistical analysis:
All values were shown as mean±Standard Error of Mean (SEM). Statistical analysis was performed using a one-way Analysis of Variance (ANOVA) followed by Dunnett’s t-test (compare all vs. control). A p-value of <0.05 was considered statistically significant, *p˂0.05 and **p˂0.01 when compared with control. All analysis was made with the statistical software Graph Pad Instat.
Results and Discussion
Phytochemical screening of the methanolic extract revealed the presence of triterpenoids, alkaloids, glycosides, phenols, tannins, flavonoids and steroids (Table 1). These phytochemical components like phenolics and flavonoids give its antioxidant activity to the plant. The methanolic extract of Ipomoea hederifolia was found to have a total phenolic content of 101.28±0.9238 mg of Gallic Acid Equivalent per gram (GAE/g). The high concentration of phenolic compounds in Ipomoea hederifolia explains why it has such a strong ability to adsorb and neutralize free radicals. The high concentration of phenolic content of leaves greatly enhances their antioxidant qualities[17]. The total flavonoid content of the leaves of Ipomoea hederifolia was expressed as mg of Quercetin Equivalent per gram (QE/g) and was found to be 14.91±0.672 QE/g of the extract.
| S. No | Phytochemical test | Methanolic extract of Ipomoea hederifolia Linn |
|---|---|---|
| 1 | Alkaloids | + |
| 2 | Glycosides | + |
| 3 | Phenols and tannin | + |
| 4 | Flavonoid | + |
| 5 | Steroids | + |
| 6 | Triterpenoids | + |
Note: + indicates the presence of the phytoconstituents
Table 1: Phytochemical Screening of the Methanolic Extract of Ipomoea hederifolia LINN
Research has demonstrated that flavonoids are exceptionally effective at scavenging a wide range of oxidizing molecules, such as singlet oxygen and various free radicals [18].
The methanolic extract's percentage of DPPH radical inhibition was compared to that of standard ascorbic acid. The extract showed concentrationdespendent increase in activity. There was a noticeable increase in the percentage inhibition of radicals with dose. The standard and the extracts' IC50 values were ascertained. Ascorbic acid was the standard, with an IC50 of 18.30 μg/ml, whereas Ipomoea hederifolia's was measured at 20.33087 μg/ml (fig. 1).
The Ipomoea hederifolia extract can donate hydrogen to a free radical, removing the unpaired electron that is responsible for the radical's reactivity, according to the DPPH assay. This assay is used to evaluate natural compounds' scavenging abilities. As the DPPH assay proceeds, the color changes from purple to yellow, indicating the presence of antioxidants[14]. Antioxidants are thought to have an impact on DPPH because of their capacity to donate hydrogen[19]. This shows Ipomoea hederifolia exhibits antiradical activity; however the standard shows higher DPPH activity compared to the standard.
The experiment was carried out over a concentration range of 20-60 μg/ml for hydroxyl scavenging activity. The Ipomoea hederifolia methanolic extract's and ascorbic acid's standard's IC50 values were found to be 104.9 μg/ml and 16.88 μg/ml, respectively. Higher concentrations led to a progressive increase in inhibition (fig. 2). Because of its high reactivity, the hydroxyl radical seriously damages molecules in its vicinity. By stimulating the production of hydroxyl radicals with ascorbic acid-EDTA, the scavenging activity of these compounds was evaluated. In the presence of DMSO, the oxidation process causes the generation of hydroxyl radicals, which results in the formation of formaldehyde. By treating the reaction product with NASH reagent, hydroxyl radicals can be easily detected[15]. The plant’s extract possessed moderate hydroxyl radical scavenging activity.
The reducing power of methanolic extract and standard (ascorbic acid) at 25 μg/ml were found to be 0.183±0.002 and 0.396±0.001 respectively. In reducing power the absorbance of the extract kept on increasing as the concentration increases (fig. 3). This method depends on the extract's ability to change the color from yellow to dark green during the reaction, which indicates that Fe3+ can be converted to Fe2+. One of the most important markers of a compound's possible antioxidant activity is its ability to reduce[20].
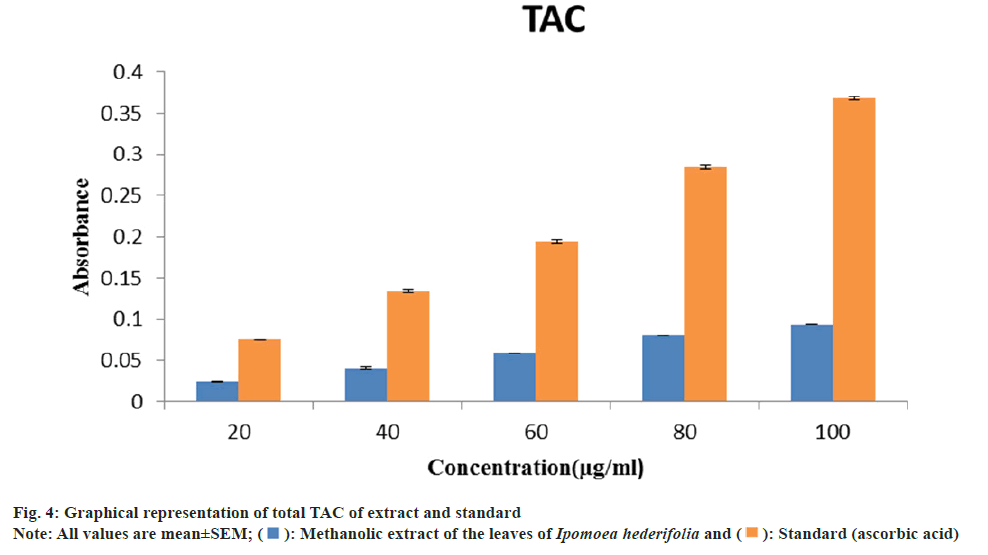
TAC of methanolic extract and ascorbic acid at 100 μg/ml were 0.094±0.0002 and 0.368±0.002 respectively (fig. 4). The methanolic extract from Ipomoea hederifolia was tested for TAC using the phosphomolybdenum method. The reduction of phosphate-molybdenum(VI) to phosphate molybdenum(V) is the basis for this assay. The extract exhibits an increase in its overall antioxidant capacity as concentration rises. The extract's and the standard's increased absorbance indicates a significant level of overall antioxidant activity[21]. The phenolic and flavonoid content of the Ipomoea hederifolia methanolic extract, in particular, may be responsible for its overall antioxidant capacity.
Acknowledgements:
I would like to express my gratitude to the Head of the Department of Pharmacy and the Director of RIPANS for their assistance in providing all the facilities required to ensure the efficient and effective completion of this research project.
Conflict of interests:
The authors declared no conflict of interests.
References
- Namiki M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr 1990;29(4):273-300.
[Crossref] [Google Scholar] [PubMed]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408(6809):239-47.
[Crossref] [Google Scholar] [PubMed]
- Mahdi BM, Jothy SL, Latha LY, Yeng CY, Sasidharan SS. Antioxidant activity of methanolic extracts of different parts of Lantana camara. Asian Pac J Trop Biomed 2012;2(12):960-5.
[Crossref] [Google Scholar] [PubMed]
- Castañeda-Gómez JF, Leitão SG, Pereda-Miranda R. Hederifolic acids AD, hepta and hexasaccharides from the resin glycosides of Ipomoea hederifolia. Phytochemistry 2023;211:113689.
[Crossref] [Google Scholar] [PubMed]
- Sawmliana M. The Book of Mizoram plants 2nd ed. Aizawl: Zakhuma;2013. p. 144-6.
- Pandurangan A, Rana K. A mini review on chemistry and biology of Ipomoea hederifolia Linn.(Convolvulaceae). Glob J Pharm Educ Res 2015;4(1-2).
- Hossain MM, Uddin MS, Baral PK, Ferdus M, Bhowmik S. Phytochemical screening and antioxidant activity of Ipomoea hederifolia stems: A potential medicinal plant. Asian J Nat Prod Biochem 2022;20(2).
- Jenett-Siems K, Schimming T, Kaloga M, Eich E, Siems K, Gupta MP, et al. Pyrrolizidine alkaloids of Ipomoea hederifolia and related species. Phytochem 1998;47(8):1551-60.
- Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem 2014;2(5):115-9.
- Mendoza N, Silva EM. Introduction to phytochemicals: secondary metabolites from plants with active principles for pharmacological importance. Phytochemicals: Source of antioxidants and role in disease prevention. 2018;25:1-5.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. Nirali Prakashan, Pune (India) 2012;6:3-6.25.
- Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 2013;3(8):623-7.
[Crossref] [Google Scholar] [PubMed]
- Mathur R, Vijayvergia R. Determination of total flavonoid and phenol content in Mimusops elengi Linn. Int J Pharm Sci Res 2017;8(12):5282-5.
- Rahman MM, Islam MB, Biswas M, Khurshid AH. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes 2015;8:1-9.
[Crossref] [Google Scholar] [PubMed]
- Pavithra K, Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci Hum Wellness 2015;4(1):42-6.
- Hossain MA, Shah MD. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab J Chem 2015;8(1):66-71.
- Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. ScientificWorldJournal 2020;9(2):45-59.
[Crossref] [Google Scholar] [PubMed]
- Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1998;56(11):317-33.
[Crossref] [Google Scholar] [PubMed]
- Baumann J, Wurn G, Bruchlausen FV. Prostaglandin synthetase inhibiting O 2-radical scavenging properties of some flavonoids and related phenolic compounds, naunyn, schmiedebergs. 1979;27-32.
- Chung YC, Chang CT, Chao WW, Lin CF, Chou ST. Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem 2002;50(8):2454-8.
[Crossref] [Google Scholar] [PubMed]
- Prasad KN, Yang B, Yang S, Chen Y, Zhao M, Ashraf M, et al. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem 2009;116(1):1-7.