- *Corresponding Author:
- A.Anand
Department of Pharmaceutics, Hygia Institute of Pharmaceutical Education and Research, Prabandh Nagar, Lucknow, Uttar Pradesh 226020, India
E-mail: anubhavanand2000@gmail.com
| Date of Received | 07 January 2022 |
| Date of Revision | 13 December 2022 |
| Date of Acceptance | 14 May 2024 |
| Indian J Pharm Sci 2024;86(3):860-871 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Simvastatin is classified as a biopharmaceutics classification system class II drug. Emulgel is a superior alternative for biopharmaceutics classification system class II drugs with low solubility and high permeability. For emulgel preparation, the solubility of simvastatin was assessed in sesame, groundnut and olive oil. The maximum solubility of simvastatin is found in sesame oil (21.06 mg/ml). Simvastatin emulgel was prepared and optimized using a three-factor, three-level Box-Behnken design. For optimization, concentrations of carbopol 934, sesame oil and surfactant (Tween 80) were selected as independent variable and viscosity, spreadability and in vitro drug release was selected as dependent variables, which are critical components of any semisolid formulation, as well as patient compliance. The optimized formulation was evaluated for viscosity, spreadability, pH, drug content and ex vivo release. The optimized formulation had 6875±531.21 mPas of viscosity, spreadability of 10.1446±0.31 gcm/s and in vitro drug release of 40.75±0.42 %, respectively. Ex vivo release investigations revealed that 42.15±0.32 % of simvastatin was released in 24 h. The study results demonstrated that the simvastatin emulgel might be an alternative the conventional topical administration form.
Keywords
Emulgel, simvastatin, Box-Behnken design, hydrophobic drug, sesame oil
Gels are the three Dimensional (3D) polymeric matrix in which large volumes of aqueous or hydroalcoholic liquids are entrapped[1]. Gel formulations often allow for faster pharmaceutical release than traditional ointments and lotions. Despite the numerous advantages of gels, one major disadvantage is the difficulty in administering hydrophobic drugs[2-4]. Emulgels were designed to help people get over these limitations. Emulgels are dosage forms made up of a mixture of gels and emulsions[5]. Emulgel is a superior alternative for Biopharmaceutics Classification System (BCS) class II drugs with low solubility and high permeability[6,7]. Emulgel is thixotropic, greaseless, easily spreadable, removable, emollient, nonstaining, water-soluble, biofriendly and pleasing in appearance, all of which contribute to patient adequacy[8].
Simvastatin ([(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)- 4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl- 1,2,3,7,8,8a-hexahydronaphthalen-1-yl]2,2- dimethylbutanoate) has also been proven to have antibacterial activity and the ability to reduce Grampositive bacteria[9-11], making it a viable alternative to some of the more well-known antibiotics[12,13]. The study's objective was to develop a topical simvastatin emulgel to improve drug loading and sustained release. A response surface approach (Box-Behnken design) was employed in this study to construct an optimal emulgel system and to investigate the impact of formulation factors on the properties of the developed emulgel system.
Materials and Methods
Materials:
Simvastatin was obtained as a gift sample from Biodeal Pharmaceuticals Pvt. Ltd., Carbopol 934 was obtained from Central drug house Pvt. Ltd. All other substances were of analytical grade and had not been modified in any way.
Methods:
Solubility study: The solubility of simvastatin in oils (sesame, arachis and olive) was determined by the shake flask method. The oils (5 ml) were kept in the vials and an excess of simvastatin was added to the vials. The vials were capped, shaken on a vortex shaker and kept for 48 h[14]. Mixtures were then centrifuged at 5000 rpm for 15 min at 25° to remove the undissolved simvastatin. The supernatant thus obtained was separated and adequately diluted with ethanol and estimated for the drug by a Ultraviolet (UV) spectrophotometer at 238 nm[15].
Preperation of emulgel:
The formula of the emulgel is shown in Table 1. The aqueous and oily phase was prepared separately. The oil phase was prepared by dissolving Tween 80 and drug in sesame oil, while methylparaben, propylparaben and glycerine were dissolved in distilled water to make the aqueous phase. Both the phases were heated individually at 70°-80° for 20 min; then oily phase was added to the aqueous phase[16] by stirring on a magnetic stirrer and allowed to cool down to room temperature to get an emulsion. The emulsion was dispersed in the gel base, prepared separately by dispersing the carbopol 934 in purified water and stirred continuously by magnetic stirrer to establish the gel phase of the formulations[17]. A 10 % Triethanolamine (TEA) solution was added dropwise to adjust pH 6.8 and produce an emulgel[18].
| S No. | Carbopol 934 (% w/v) | Sesame oil (% w/v) | Tween 80 (% w/v) | Glycerine (ml) | Methyl paraben (g) | Propyl paraben (g) | Triethanolamine | Distilled water q.s. (ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.5 | 5 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 2 | 1 | 5 | 3 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 3 | 0.5 | 5 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 4 | 0.5 | 10 | 1 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 5 | 1 | 10 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 6 | 1 | 10 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 7 | 1.5 | 10 | 3 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 8 | 0.5 | 15 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 9 | 0.5 | 10 | 3 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 10 | 1 | 15 | 1 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 11 | 1 | 15 | 3 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 12 | 1.5 | 15 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 13 | 1 | 10 | 2 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 14 | 1.5 | 10 | 1 | 0.6 | 0.05 | 0.05 | q.s | 100 |
| 15 | 1 | 5 | 1 | 0.6 | 0.05 | 0.05 | q.s | 100 |
Note: q.s.: Quantity sufficient
Table 1: Formulation of SIMVASTATIN EMULGEL
Optimization of emulgel formulation:
Box-Behnken design was employed to evaluate the impact of independent factors on the responses to establish the optimal emulgel formulation. Carbopol 934 (Polymer), sesame oil (Oil), and Tween 80 (surfactant) concentrations were the independent variables. Viscosity (Y1), Spreadability (Y2), and in vitro drug release (Y3) were the dependent responses. The concentrations of the independent variables are shown in Table 2. The emulgel formulation was optimized using a three-factor, three-level (33) design[19]. The software predicted a design with 15 experimental runs, three of which are central values as shown in Table 3.
| Independent variables | Levels | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| Carbopol 934 % w/v (A) | 0.5 | 1 | 1.5 |
| Oil % w/v (B) | 5 | 10 | 15 |
| Surfactant % w/v (C) | 1 | 2 | 3 |
Table 2: Independent Variables and their Levels for Box- Behnken Design of EMULGEL
| Run | Carbopol 934 (% w/v) | Oil (% w/v) | Surfactant (% w/v) |
|---|---|---|---|
| 1 | 1 | -1 | 0 |
| 2 | 0 | -1 | 1 |
| 3 | -1 | -1 | 0 |
| 4 | -1 | 0 | -1 |
| 5 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 |
| 7 | 1 | 0 | 1 |
| 8 | -1 | 1 | 0 |
| 9 | -1 | 0 | 1 |
| 10 | 0 | 1 | -1 |
| 11 | 0 | 1 | 1 |
| 12 | 1 | 1 | 0 |
| 13 | 0 | 0 | 0 |
| 14 | 1 | 0 | -1 |
| 15 | 0 | -1 | -1 |
Table 3: Three Factor three level box BEHNKEN design in Coded Value
Evaluation parameters of emulgel:
Organoleptic character: Organoleptic properties such as colour, appearance and homogeneity of the prepared emulgel were visually evaluated.
Viscosity: The viscosity of the formulations was determined using a Brookfield Viscometer (RV viscometer)[20]. The spindle (#5) was placed in the emulgel's centre, ensuring it did not touch the bottom of the beaker and revolved for 10 min at 10 rpm. The viscosity was calculated and reported. An average of three readings was used to determine the viscosity of the emulgel.
Spreadability test: Khullar et al.[21] provided a method for determining the spreading coefficient. It has built out of a wooden block with a pulley on one end. The spreading coefficient was calculated using the emulgel's 'Slip and Drag' features. A glass slide was mounted on the wooden block. An excess amount of emulgel (about 2 g) was placed on the ground slide. Another slide, attached with thread, was placed over the fixed one. The thread was passed over the pulley and another end of the thread held a pan. A 2 g weight was placed initially over the two slides to form a uniform film between the slides. After 5 min, the weight was removed and was put on the pan to move the upper slide, and the time (in s) needed by the top slide to traverse a maximum distance of 5 cm was noted[22]. The spreading coefficient was calculated by using the formula:
S=M×L/T
Where M, is a weight put on the pan attached to the upper slide, L is for the length covered by the glass slides, T is the time taken by the slide to cover the distance L.
pH determination: At room temperature, the pH of the sample was determined using a digital pH metre (Thermo Scientific). The emulgel was suitably diluted with purified water and allowed to sit for 2 h. The pH was checked directly by dipping the electrode into the gel and allowed to equilibrate[23], and then the pH was measured by calibrated pH meter.
Extrudability test (tube test)[24]: The weight needed to extrude a 0.5 cm emulgel ribbon from a lacquered collapsible aluminium tube in 10 s is the basis for the extrudability test. A collapsible aluminium tube was filled with the optimum emulgel mixture and the end was crimped shut to seal it. The substance was forced through the tubes and the emulgel's extrudability was verified. The following formula was then used to determine the extrudability.
Extrudability=Weight applied to extrude emulgel from tube (g)/area (cm2)
Swelling index[25]: It is determined by taking 1 g of emulgel in a porous aluminum foil and mixed with 0.1 N NaOH kept in a 50 ml beaker. Then samples are withdrawn at different time intervals and kept for drying and it is reweighed.
Swelling index=Wt-W0/Wt×100
Where, Wt=Weight of swollen emulgel after time ‘t’; W0= Weight of emulgel at zero time.
Microscopy of emulgel: The emulgel was placed on the glass slide and the droplet size and morphology of the emulgel was studied by microscope with the camera (Radical [RXL-4T]) at a magnification of 10X[26].
Drug content: Drug content was determined by dissolving a 1 g of emulgel in 10 ml of ethanol, stirring with a magnetic stirrer until the emulgel was dissolved entirely. The solution was centrifuged at 5000 rpm for 10 min and the clear supernatant was taken and diluted suitably. The absorbance was recorded at λmax 238 nm with a double beam UV spectrophotometer (Labindia).
Percentage drug content=Drug content (mg)/Label claim×100
In vitro release test:
In vitro drug release studies were carried out using a Franz Diffusion (FD) cell with a dialysis membrane (HIMEDIA LA 395-10MT). The phosphate buffer pH 7.4 solution containing 0.5 % w/v of sodium dodecyl sulphate was used as a dissolution media. This buffer solution was placed into the receptor compartment of the diffusion cell. The membrane previously soaked in phosphate buffer pH 7.4 for 24 h was carefully clamped on between the donor and receiver chamber of the diffusion cell. The entire assembly was placed on a magnetic stirrer. The solution on the receptor side was continually stirred while the temperature was preserved at 37°. To maintain sink condition, an aliquot was withdrawn at different intervals and replaced with equal amounts of fresh dissolution media. The sample was diluted with phosphate buffer. These samples were analyzed on UV visible spectrophotometer (Labindia) at 238 nm and the cumulative percent drug release was calculated.
Ex vivo release test:
Ex vivo drug release was studied by FD cell, using excised rat skin. The skin was fastened amid the donor and receptor chambers of the Franz cell. 1 g of emulgel was spread over to the rat skin. Phosphate buffer pH 7.4 solution containing 0.5 % w/v of sodium dodecyl sulphate was used as a dissolution media. This buffer solution was filled up in the receptor compartment, kept at 37° and stirred at 50 rpm. 3 ml of aliquot was withdrawn from the receptor compartment with the help of an extra-long needle syringe via sampling port at prespecified time intervals. For maintaining the sink condition, an equal amount of phosphate buffer was introduced back to the receptor compartment. Samples were analyzed at 238 nm for simvastatin using a UV-visible spectrophotometer[27].
Stability test:
Emulgel was supplied in aluminium tubes that could be folded up (5 g). 3 samples in a set were stored under different settings and subjected to tests for a month at three different temperatures; 5°/60 % RH, 25°/60 % RH and 30°/65 % RH. Every 10 d, samples are collected in accordance with International Council for Harmonisation (ICH) requirements and their colour, viscosity, spreadability, pH and medication concentration are assessed.
Results and Discussion
The solubility data obtained for simvastatin in various oil media was used to select oil for emulgel. The solubility of simvastatin in different oil was shown in Table 4 and the maximum solubility was found in sesame oil. Oil with a maximum solubility of simvastatin was used in the emulgel preparation because it enhances the drug loading in the emulgel.
| S No. | Oil | Solubility (mg/ml) |
|---|---|---|
| 1 | Sesame oil | 21.06±0.99 |
| 2 | Arachis oil | 20.92±0.41 |
| 3 | Olive oil | 17.53±0.79 |
Table 4: Solubility Of SIMVASTATIN in Oil
All the responses (dependent variables) observed for the 15 runs were simultaneously fitted to the quadratic response surface model using the trial version of Design-expert® 13 (StatEase®) software. The observed responses/dependent variables for the emulgel formulation are shown in Table 5. The physicochemical character of the emulgel is a crucial issue to consider before starting the fabrication process, especially when it comes to topical drug delivery.
| Run | Carbopol 934 (% w/v) | Oil (% w/v) | Surfactant (% w/v) | Viscosity (mPas) | Spredability (gcm/s) | Drug release (%) |
|---|---|---|---|---|---|---|
| 1 | 1.5 | 5 | 2 | 10069 | 14.59 | 40.23 |
| 2 | 1.0 | 5 | 3 | 4998 | 2.07 | 41.67 |
| 3 | 0.5 | 5 | 2 | 6643 | 4.42 | 38.92 |
| 4 | 0.5 | 10 | 1 | 5298 | 1.93 | 36.32 |
| 5 | 1.0 | 10 | 2 | 7554 | 7.3 | 35.72 |
| 6 | 1.0 | 10 | 2 | 12281 | 1.86 | 32.81 |
| 7 | 1.5 | 10 | 3 | 12464 | 2.08 | 30.23 |
| 8 | 0.5 | 15 | 2 | 3663 | 0.686 | 27.12 |
| 9 | 0.5 | 10 | 3 | 4165 | 0.69 | 28.67 |
| 10 | 1.0 | 15 | 1 | 15911 | 2.24 | 26.01 |
| 11 | 1.0 | 15 | 3 | 11099 | 0.335 | 21.32 |
| 12 | 1.5 | 15 | 2 | 21970 | 1.11 | 20.12 |
| 13 | 1.0 | 10 | 2 | 4714 | 1.18 | 23.43 |
| 14 | 1.5 | 10 | 1 | 16753 | 1.24 | 21.43 |
| 15 | 1.0 | 5 | 1 | 9870 | 4.12 | 30.14 |
Table 5: Independent Variable and their Responses
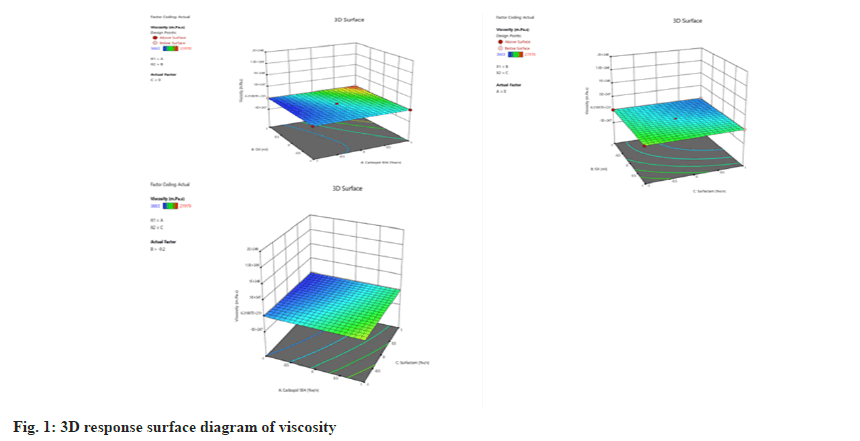
The viscosity is the most critical factor in emulgel formulation. The viscosity prevents the coalescence and formation of the large droplet (instability in the emulsion) in the case of emulsion. The prepared emulgels have a viscosity of 4165 to 16753 mPas. The model F value of 6.16 indicates that the model is significant in this case. The parameter substantially affects the design if the model p value is less than 0.05. The model p value was found to be 0.0296 (<0.05), indicating that the parameter has a significant impact on the experimental design. Additionally, there is only a 2.96 percent probability that an F value will become significant due to noise.
Viscosity = 8183+5185.875A+2632.87B-1888.25C+3720.25AB-789AC+15BC+801.875A2 +1601.375B2+685.125C2
The p value less than 0.05 indicates the significance of model terms. A, B and AB were significant model terms (Table 6) because the p value was 0.0023, 0.0343 and 0.0344. Carbopol 934 (A) and oil (B) have a significant effect on viscosity in the current design (fig. 1). In emulgel formulation, carbopol is utilized as a gelling agent and any change in carbopol concentration reflects in the viscosity of the emulgel. Oils are also viscous liquids, so if there is an increase in oil percentage, it increases viscosity. Carbopol and oil have also shown a positive interaction effect on viscosity, i.e., the viscosity is further increased due to the interaction of carbopol and oil. The positive terms indicate that the viscosity increases on increasing carbopol concentration and oil percentage.
| Viscosity | p-values | |
|---|---|---|
| Intercept | 8183 | 0 |
| A | 5185.87 | 0.0023 |
| B | 2632.87 | 0.0343 |
| C | -1888.3 | 0.0932 |
| AB | 3720.25 | 0.0344 |
| AC | -789 | 0.5675 |
| BC | 15 | 0.9912 |
| A2 | 801.875 | 0.5764 |
| B2 | 1601.38 | 0.2865 |
| C2 | 685.125 | 0.6316 |
Table 6: Coefficients Table for Viscosity
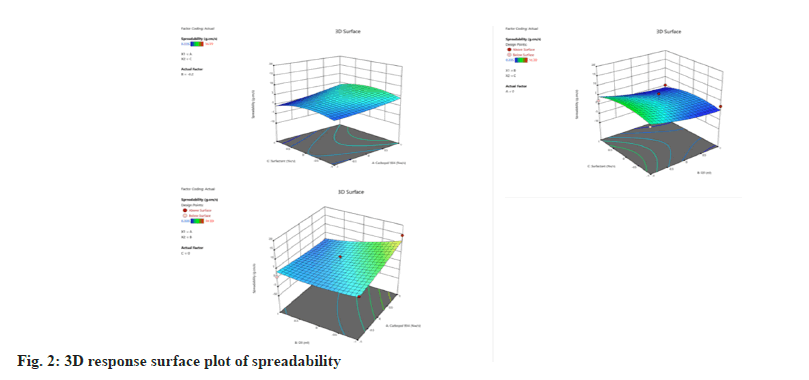
The spreadability test demonstrated how easily the emulgel could be spread on the affected area with just a little shear. The spreadability of the emulgel formulation was ranged from 0.335-14.59 gcm/s indicating that the formulation is non-greasy and smooth. A quadratic model of polynomial analysis was used to analyze spreadability values.
Spreadability=3.44667+1.41175A-2.60362B- 0.544375C-2.4365AB+0.52AC+0.03625BC+0.52 4292A2+1.23054B2-2.48596C2
The specified model terms are significant when the p values are less than 0.05. There are no significant model terms in the case of spreadability (Table 7), and it implies that there is no factor that significantly affects the spreadability. The 3D surface diagram of the spreadbility was shown in fig. 2.
| Spreadability | p values | |
|---|---|---|
| Intercept | 3.44667 | 0 |
| A | 1.41175 | 0.2991 |
| B | -2.60362 | 0.0858 |
| C | -0.544375 | 0.6739 |
| AB | -2.4365 | 0.2167 |
| AC | 0.52 | 0.7751 |
| BC | 0.036525 | 0.984 |
| A2 | 0.524292 | 0.7819 |
| B2 | 1.23054 | 0.5233 |
| C2 | -2.48596 | 0.2245 |
Table 7: p Value for the Coefficient in Spreadability
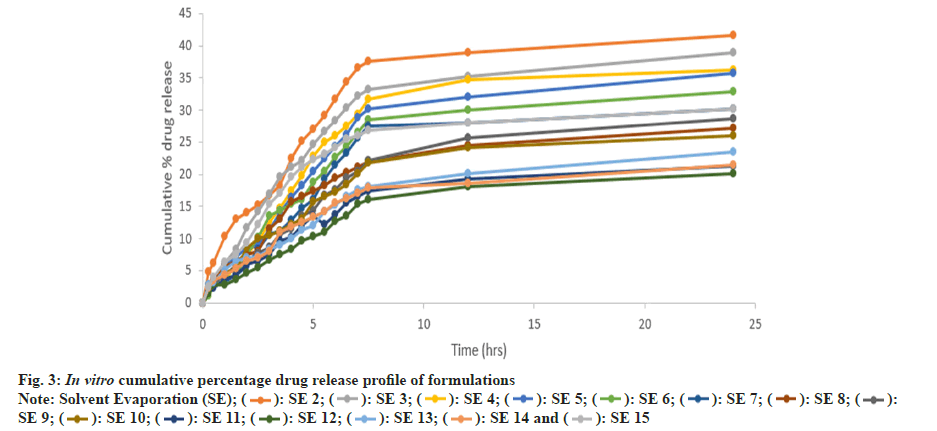
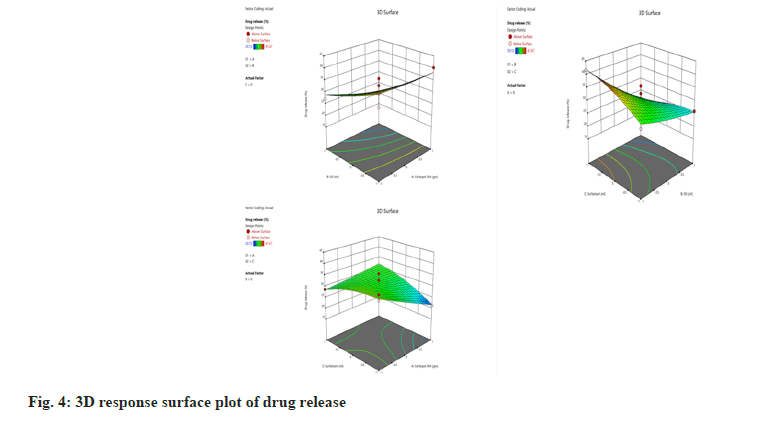
The drug release is the extensive parameter that could differentiate emulgel. The drug release of the developed emulgels was in range of 20.12 and 41.67 % (fig. 3). This formulation showed the sustained type of drug release over an extended period. The 3D surface plot of in vitro drug release was shown in fig. 4.
Drug release=30.65-2.38A–7.05B+0.9988C– 2.08AB+4.11AC–4.05BC+0.1608A2+0.7833B2– 1.65C2
Table 8, depicting the coefficient value shown that the oil percentage significantly affected the drug release, by the p value of 0.0068 (<0.05). As per the equation, the value of oil (B) preceded the negative sign, which indicates that an increase in oil percentage causes a decrease in the drug release. It can be attributed to the lipophilic character of the drug. Because the drug is lipophilic; therefore, it has an affinity towards the oil phase, and it slowly partitions into the aqueous phase of emulsion and gel phase of emulgel.
| Source | Sum of squares | Df | Mean square | F value | p value |
|---|---|---|---|---|---|
| Model | 614.7 | 9 | 68.3 | 3.39 | 0.0961ns |
| A-Carbopol 934 | 45.22 | 1 | 45.22 | 2.24 | 0.1944 |
| B-Oil | 397.48 | 1 | 397.48 | 19.73 | 0.0068 |
| C-Surfactant | 7.98 | 1 | 7.98 | 0.3961 | 0.5568 |
| AB | 17.26 | 1 | 17.26 | 0.8568 | 0.3971 |
| AC | 67.65 | 1 | 67.65 | 3.36 | 0.1264 |
| BC | 65.77 | 1 | 65.77 | 3.26 | 0.1306 |
| A2 | 0.0955 | 1 | 0.0955 | 0.0047 | 0.9478 |
| B2 | 2.27 | 1 | 2.27 | 0.1124 | 0.751 |
| C2 | 10.07 | 1 | 10.07 | 0.4999 | 0.5111 |
| Residual | 100.74 | 5 | 20.15 | ||
| Lack of fit | 18.24 | 3 | 6.08 | 0.1474 | 0.9229ns |
| Pure error | 82.5 | 2 | 41.25 | ||
| Cor total | 715.44 | 14 |
Note: Not significant
Table 8: Coefficient Tables for Drug Release
Optimization of emulgel formulation:
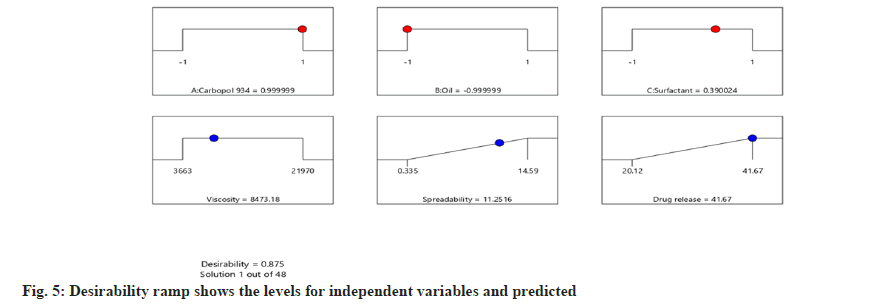
Following the completion of the experiments, the data was entered into design expert software, along with the desirability of the responses (dependent variable). Based on the run's replies, the software projected the values of the independent variable indicated in Table 9. The software predicted the values of dependent variables depending on the response values (fig. 5). The optimized formulation with predicted values of independent variables was prepared and evaluated. The response/dependent variables value was shown in Table 10. The resulting optimum formulation had an average of those three runs found for viscosity 6875±531.21 mPas, spreadability of 10.1446±0.31 gcm/s and drug release of 40.75±0.42 %, showing that the ideal formula was reasonably stable.
| Factor | Name | Level | Low level | High level | Standard deviation | Coding |
|---|---|---|---|---|---|---|
| A | Carbopol 934 | 1 | -1 | 1 | 0 | Actual |
| B | Oil | -1 | -1 | 1 | 0 | Actual |
| C | Surfactant | 0.39 | -1 | 1 | 0 | Actual |
Table 9: Predicted Coded Value of Independent Variable
The organoleptic properties of emulgels are shown in Table 10. The formulations were homogeneous, white in colour, non-greasy, pleasant to the touch and had no phase separation. The extrudability of a pharmaceutical formulation is a critical parameter because it can influence the manufacturing process and quality of finished product. The formulation should have right rheological properties to be successfully extruded. The extrudability of the formulations are shown in Table 10 and were ranged from 10.1±0.05 to 16.4±0.06 g/cm2. The higher extrudability was found in formulation containing higher percentage of polymer. The optimized formulation exhibited the 12.7±0.07 g/ cm2.
| Formulation | Colour | Appearance | Extrudability (g/cm2) |
| F1 | White | Homogenous | 15.1±0.02 |
| F2 | White | Homogenous | 14.1±0.07 |
| F3 | White | Homogenous | 10.2±0.01 |
| F4 | White | Homogenous | 12.6±0.07 |
| F5 | White | Homogenous | 11.4±0.04 |
| F6 | White | Homogenous | 12.2±0.06 |
| F7 | White | Homogenous | 15.8±0.05 |
| F8 | White | Homogenous | 10.5±0.02 |
| F9 | White | Homogenous | 11.1±0.01 |
| F10 | White | Homogenous | 10.1±0.05 |
| F11 | White | Homogenous | 11.3±0.02 |
| F12 | White | Homogenous | 16.4±0.06 |
| F13 | White | Homogenous | 10.3±0.01 |
| F14 | White | Homogenous | 16.1±0.06 |
| F15 | White | Homogenous | 10.2±0.02 |
| OF | White | Homogenous | 12.5±0.07 |
Note: OF: Optimized Formulation
Table 10: Extrudability Data of Test Formulation and Optimized Formulation
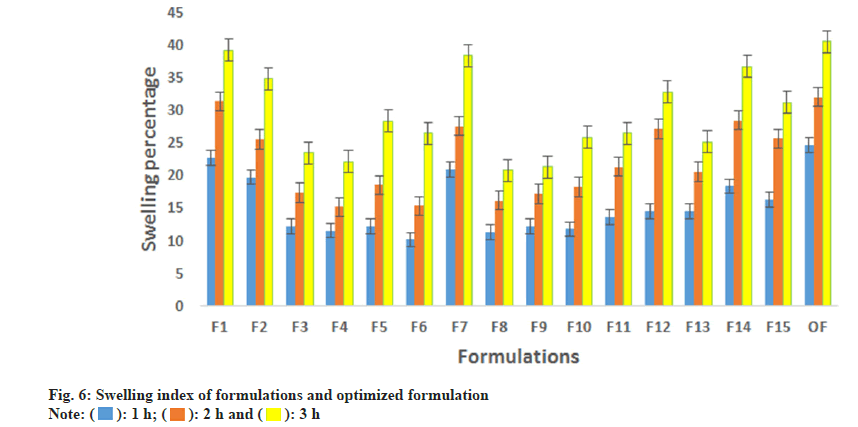
The swelling index is a critical parameter in formulations involving polymers that may undergo swelling. The drug release from the formulations can be controlled through the swelling of the polymer materials. Fig. 6, shows the percentage swelling in the formulation. The maximum swelling was observed in the formulation F1 (39.2 %) followed by F7 (38.4 %). Both formulations contain high polymer concentration (1.5 % w/v). With spindle 05, the emulgel was rotated at 10 rpm for 10 min. The optimized formulation's viscosity was found to be 6875±29 mPas which was in desired range.
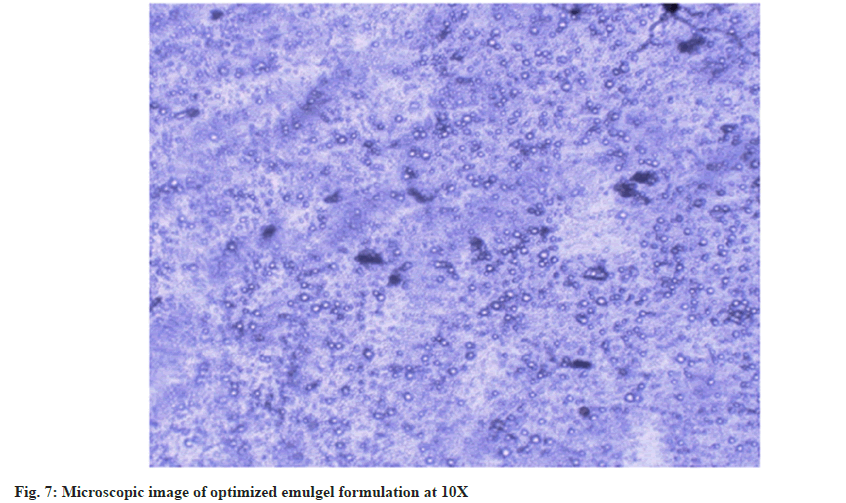
Spreadability reveals how easily the emulgel can be dispersed with a modest amount of shear[28]. The spreadability of the emulgel is highly requisite since it reveals how it behaves when it gets out of the tube[20]. The improved formulation's spreadability was found to be 10.15±0.30 gcm/s. Tiny oil globules dispersed in the polymer matrix were visible in the microscopic image (fig. 7) of the emulgel formulation. The globules are all the same size and are dispersed equally throughout the matrix. The average droplet size of the emulsion is about 3±0.16 μm. A digital pH metre was used to determine the pH of the optimized formulation. The formulation had a pH of 6.85±0.01. The drug content of the prepared emulgel was estimated. The drug content in optimized emulgel was realized to be 98.26±0.31 %.
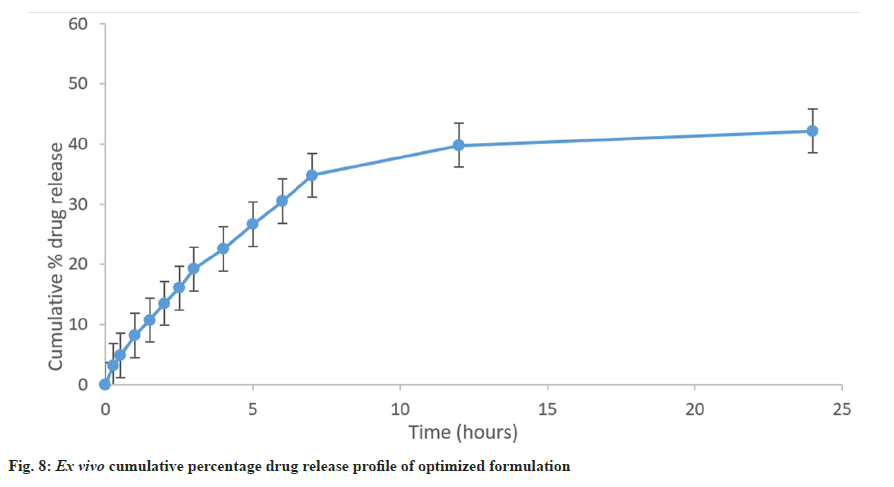
Ex vivo release studies were performed to understand the drug release behaviour through the skin. The release of the drug from the emulgel formulation was 40.75±0.42 % over 24 h (fig. 8). The initially slightly high drug release was observed due to the drug partitioned in the gel phase. After that, the drug release was slowed down due to the slow partitioning of lipophilic drugs from the oil phase of the emulsion to the gel phase (Table 11).
| Parameters | Values |
|---|---|
| Viscosity | 6875±531 mPaS |
| Spreadability | 10.14±0.30 gcm/s |
| Percentage drug release | 40.75±0.42 |
| pH | 6.85±0.01 |
Table 11: Response Data of Optimized Formulation
In conclusion, Box-Behnken design was used to optimize a carbopol 934, oil, surfactant loaded with simvastatin intended for the topical treatment of wounds in the current investigation. The improved formulation was obtained and put through a series of physicochemical tests, including rheological tests, spreading coefficient tests, drug content tests, and an ex vivo release study on rat skin. The prepared emulgel revealed a maximum release of 40.75±0.42 % in 24 h in an ex vivo investigation. Furthermore, the microscope image reveals that the globules in the matrix are consistent in size and equally dispersed. As a result, simvastatin emulgel can be employed as a topical drug delivery system.
Conflict of interest:
The authors declare no conflict of interest.
References
- Kaur LP, Garg R, Gupta GD. Topical gels: A review. Res J Pharm Technol 2010;3(1):17-24.
- Surekha M, Khan PM, Reddy GG, Tabassum SA, Wilson KV, Siddiqua SA. Emulgel-an overview. World J Pharm Res 2019;8(4):394-406.
- Redkar MR, Patil SV, Rukari TG. Emulgel: A modern tool for topical drug delivery. World J Pharm Res 2019;8(4):586-97.
- Vats S, Saxena C, Easwari TS, Shukla VK. Emulsion based gel technique: Novel approach for enhancing topical drug delivery of hydrophobic drugs. Int J Pharm Res Scholars 2014;3(2):649-60.
- Khalid SA, Kumar BP, Abhinov T. Formulation and in vitro evaluation of aceclofenac loaded topical emulgel. Indian J Res Pharm Biotechnol 2014;2(6):1487.
- Saini M, Singh S, Verma S. Formulation and evaluation of an emulgel of a BCS class II drug. Int J Pharm Res 2019;11(3):1-7.
- Ismail Shehada MB, Usman S, Akram M, Usman A. Innovative approaches to enhance dissolution rate of a hydrophobic drug glimepiride. J Pharm Pharmacol 2020;8(1):1-10.
- Sah SK, Badola A, Nayak BK. Emulgel: Magnifying the application of topical drug delivery. Indian J Pharm Biol Res 2017;5(1):25-33.
- Khan AR, Riaz M, Bin Abdulhak AA, Al-Tannir MA, Garbati MA, Erwin PJ, et al. The role of statins in prevention and treatment of community acquired pneumonia: a systematic review and meta-analysis. PloS one 2013;8(1):e52929.
[Crossref] [Google Scholar] [PubMed]
- Björkhem-Bergman L, Bergman P, Andersson J, Lindh JD. Statin treatment and mortality in bacterial infections–a systematic review and meta-analysis. PloS one 2010;5(5):e10702.
[Crossref] [Google Scholar] [PubMed]
- Graziano TS, Cuzzullin MC, Franco GC, Schwartz-Filho HO, de Andrade ED, Groppo FC, et al. Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PloS one 2015;10(5):e0128098.
[Crossref] [Google Scholar] [PubMed]
- Murtaza G. Solubility enhancement of simvastatin: A review. Acta Pol Pharm 2012;69(4):581-90.
[Google Scholar] [PubMed]
- Chavhan SS, Petkar KC, Sawant KK. Simvastatin nanoemulsion for improved oral delivery: Design, characterisation, in vitro and in vivo studies. J Microencapsul 2013;30(8):771-9.
[Crossref] [Google Scholar] [PubMed]
- Munir A, Ahmad M, Malik MZ, Minhas MU. Analysis of simvastatin using a simple and fast high performance liquid chromatography-ultra violet method: development, validation and application in solubility studies. Tropic J Pharm Res 2014;13(1):135-9.
- Mahmoud H, Al-Suwayeh S, Elkadi S. Design and optimization of self-nanoemulsifying drug delivery systems of simvastatin aiming dissolution enhancement. Afr J Pharm Pharmacol 2013;7(22):1482-500.
- Patra HK, Dasgupta AK, Sarkar S, Biswas I, Chattopadhyay A. Dual role of nanoparticles as drug carrier and drug. Cancer Nanotechnol 2011;2:37-47.
[Crossref] [Google Scholar] [PubMed]
- Yadav S, Wairkar S, Invally M, Ranade S. Topical emulgel of tolnaftate with penetration enhancer: development, characterisation and antifungal activity. Indian J Med Res Pharm Sci 2017;4(10):28-35.
- Jamadar MJ, Shaikh RH. Preparation and evaluation of herbal gel formulation. J Pharm Res Edu 2017;1(2):201-4.
- Sheetlani J, Thakur SB. Preparation and enlargement of topical flurbiprofen emulgel by with xanthan gum. Int J Sci Res Eng Technol 2018;29:29-40.
- Baibhav J, Gurpreet S, Rana AC, Seema S. Development and characterization of clarithromycin emulgel for topical delivery. Int J Drug Dev Res 2012;4(3):310-23.
- Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J 2012;20(1):63-7.
[Crossref] [Google Scholar] [PubMed]
- Srivastava A, Desai S, Jain H, Meshram DB. Formulation and evaluation of fusidic acid emulgel. J Drug Deliv Ther 2020;10(3-s):169-75.
- Ahmed MM, Fatima F, Anwer MK, Ibnouf EO, Kalam MA, Alshamsan A, et al. Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection. Saudi Pharm J 2021;29(5):467-77.
[Crossref] [Google Scholar] [PubMed]
- Ambhore NP, Dandagi PM, Gadad AP, Mandora P. Formulation and characterization of tapentadol loaded emulgel for topical application. Indian J Pharm Edu Res 2017;51:525-35.
- Raju K, Sneha G, Khatoon R, Ashwini M, Shirisha G, Ajay B, et al. Formulation and evaluation of ornidazole topical emulgel. World J Pharm Pharm Sci 2019;8(07):1179-97.
- Pranali S, Charushila S, Sayali C, Namrata M. Design and characterisation of emulgel of an antifungal drug. J Pharm Sci Res 2019;11(6):2357-61.
- Burki IK, Khan MK, Khan BA, Uzair B, Braga VA, Jamil QA. Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech 2020;21:1-4.
[Crossref] [Google Scholar] [PubMed]
- Varma VN, Maheshwari PV, Navya M, Reddy SC, Shivakumar HG, Gowda DV. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm J 2014;22(6):591-9.
[Crossref] [Google Scholar] [PubMed]