- Corresponding Author:
- B. Boonyapiwat
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health, Thailand
E-mail: boontarika.b@dmsc.mail.go.th
| Date of Submission | 28 July 2010 |
| Date of Revision | 18 October 2011 |
| Date of Acceptance | 28 October 2011 |
| Indian J Pharm Sci, 2011, 73 (5): 564-568 |
Abstract
A simple HPLC method was developed and validated for the quantification of zanamivir in permeability studies using Caco-2 cell culture model. Chromatographic resolution was achieved using 98% (v/v) ultrapure water and 2% (v/v) acetonitrile as mobile phase with flow rate of 0.5 ml/min on a BDS Hypersil Cyano column (length 250 mm; internal diameter 4.6 mm; particle size 5 μm) and UV detection at 230 nm. The method was linear for the quantification of zanamivir at concentration ranging from 0.1-10 μg/ml with coefficient of determination greater than 0.999. The recovery of zanamivir was in the range of 99.76-105.08%. The relative standard deviations of the within-day precision and between-day precision were lower than 10.32 and 14.33%, respectively. The permeability of zanamivir was independent of the transport direction and zanamivir concentrations, indicating a passive transport of zanamivir across Caco-2 cells. With the absence of Ca2+ in transport medium, the permeability values of zanamivir increased 56.21 and 57.20 fold in the directions of apical to basolateral and basolateral to apical, respectively. On the basis of these results, zanamivir was found to be predominantly transported across Caco-2 monolayers via the passive paracellular pathway.
Keywords
Absorption, Caco-2, HPLC, permeability study, zanamivir
Zanamivir has been indicated to provide activity against influenza viruses [1-3]. Each year, influenza viruses continue to cause major health problems and economic loss worldwide [3,4]. Especially, A(H1N1) and H5N1 influenza viruses lead to an unacceptable number of deaths and serious concerns about global flu pandemics [1,5-7]. Influenza viruses carry two surface glycoproteins, a hemagglutinin and a neuraminidase, which are involved in the production processes for new virions [8]. Hemagglutinin binds to the cell surface by recognizing the cellular sialic acid receptor [9] Neuraminidase cleaves the terminal sialic acid residues and releases progeny virus from the surface of infected cell [10].
The World Health Organization (WHO) recommends two neuraminidase inhibitors, oseltamivir (Tamiflu® ) and zanamivir, for the treatment of A(H1N1) and H5N1 flu. Zanamivir has been reported to have activity against oseltamivir-resistant H1N1 and the H5N1 influenza viruses [7,11,12]. Unlike oseltamivir, zanamivir can not be orally administered due to its poor oral bioavailability (~2%) [8,13,14]. A study revealed that a median of 10 to 20% of an inhaled administration of zanamivir was systemically absorbed leading to low serum concentration [14]. Therefore, zanamivir has been launched to the market only in a dry powder form for inhalation [8,13].
To date, no study reports on the mechanism of zanamivir absorption via human gastrointestinal tract or a satisfactory explanation of the factors causing low bioavailability have been undertaken or published. An understanding of the mechanism of absorption would be a remarkable milestone in the development of a formulation, which could improve oral bioavailability of zanamivir. The Caco-2 cell culture model is commonly employed to study mechanisms of drug absorption in vitro [15-17]. The aim of this study was the evaluation of zanamivir transport across Caco-2 model using a validated HPLC method.
Materials and Methods
HPLC grade acetonitrile for the mobile phase used in this study was purchased from Mallinckrodt Chemicals (USA). Ultrapure water was obtained with UHQ Ultrapure Water from ELGA (UK). Hank’s balance salt solution was from Sigma- Aldrich (Germany). N-acetyl neuraminic acid was purchased from R and S PharmChem Co., Ltd. (China). Dulbecco’s modified Eagle’s medium, fetal bovine serum and L-glutamine were received from Hyclone (USA). 3-(4,5-Dimethyl-2-thiazolyl)- 2,5-diphenyl-2H-tetrazolium bromide were from Sigma-Aldrich (Germany). Oseltamivir phosphate was a gift from The Government Pharmaceutical Organization (Thailand). Oseltamivir carboxylate (OC) was obtained by the hydrolysis of oseltamivir phosphate. Zanamivir was synthesized by using modified method of Chandler and co-workers [18].
HPLC conditions
The Waters HPLC system (model Alliance 2695) consisted of a pump and autosampler, connecting with a UV Detector (model Waters 2996). The Empower QuickStart 2514 for data processing was used. The chromatographic resolution was carried out using a BDS Hypersil Cyano column (length 250 mm; internal diameter 4.6 mm; particle size 5 μm), and with UV detection at 230 nm. The guard column was the same material as the analytical column. The temperature of column was around 25°. The mobile phase was composed of 98% ultrapure water and 2% acetonitrile (v/v) with a flow rate of 0.5 ml/ min. Each sample was injected with the volume of 10 μl. A 10 mg/ml stock zanamivir solution was prepared in ultrapure water. Standard zanamivir solutions in Hank’s balance salt solution (HBSS) at a concentration range of 0.1-10 μg/ml were obtained by serial dilutions. The internal standard (IS), OC at concentration of 2 μg/ml, was added into each working solution.
Validation
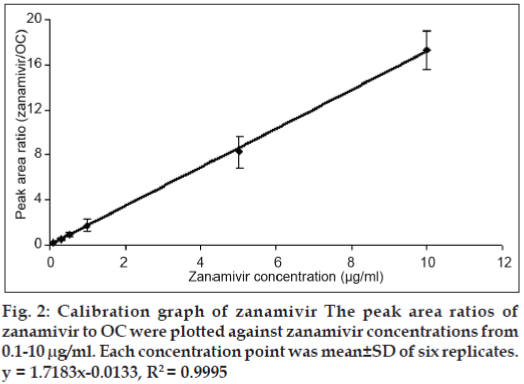
The parameters validated for the method development were sensitivity, linearity, accuracy, precision and stability. The linearity of the standard curve was analyzed in a concentration range 0.1-10 μg/ml. The existence of the broad calibration range was necessary for the type of pharmacokinetic assay intended to undertaken. The calibration curve was evaluated by peak area ratios of zanamivir to OC (IS) against zanamivir concentrations. The calibration curve was carried out in six replicates per concentration.
Three concentrations of each validation range with low, intermediate and high concentrations were evaluated. Six determinations at each concentration were analyzed. The calculated concentrations from the calibration curve were served as the experimental concentration (Cex). To check recovery, the Cex and theoretical concentration (Cth) were calculated and expressed as a percentage (Cex/Cth)×100. The withinand between-day precisions were performed by analyzing on the same day and six different days. Each concentration was carried out in six replicates.
The effects of freeze/thaw cycle on the stability of zanamivir were evaluated at low, medium and high concentrations. The tests were conducted at -20° as the freezing temperature and room temperature as thawing temperature and the samples were determined after three freeze-thaw cycles. The short-term temperature stability was performed by thawing aliquots of low, medium and high concentrations at room temperature and kept at this temperature for 36 h before analyzing. Six replicates were determined for each concentration.
Permeability and cytotoxicity study
Caco-2 cells (passage: 45-53) were grown in vent capped 75 cm2 tissue culture flasks at 37° in a humidified atmosphere of 5% CO2. The culture medium composed of Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% L-glutamine. Caco-2 cells were seeded at a density of 220,000 cells/cm2 onto 12-well Transwell® plate with an polycarbonate membrane insert at pore size of 3.0 μm. Cells were fed every other day and maintained at 37° and 5% CO2 humidified atmosphere for 21 days. The transepithelial electrical resistance (TEER) values for the integrity of the cell monolayer were measured using a Millicell-ERS volt/ohmmeter (Millipore Corp., Bedford, MA).
The cytotoxicity of zanamivir solutions at concentrations of 300 and 400 μg/ml was performed by the 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2Htetrazolium bromide (MTT) assay [19]. Caco-2 cells were seeded onto a 96 well plate at a density of 1×104 cell/well in 100 μl medium for 24 h in the incubator at 37° and 5% CO2. After 24 h incubation, growth medium in each well was replaced with fresh medium containing zanamivir solutions at final concentrations of 300 and 400 μg/ml. The plate was incubated for another 3 h. The zanamivir solutions were replaced with 100 μl MTT (0.5 mg/ml in HBSS, pH 7.4) and incubated for another 4 h at 37°. Subsequently, the MTT medium was removed and the formazan crystals dissolved in DMSO, gentle shaking for 10 min to achieve complete dissolution. The optical density (OD) was measured at 540 nm. Six replicates of each test sample were performed. Every test included a control well containing complete culture medium with cells and a blank containing complete medium without cells.
Before performing the transport studies, Caco-2 cells were washed twice with warm HBSS. After the wash, the plates were pre-incubated at 37° for 45 min. After the wash, the TEER values were measured and the inserts with TEER values above 600 ohm. cm2 were used in the studies. Zanamivir solutions at concentrations of 300 and 400 μg/ml were employed in the study. HBSS was replaced with 0.5 and 1.5 ml of zanamivir solutions either into the apical or into the basolateral compartment with respect to the cell layer to study transport from apical to basolateral (AP-BL) and bi-directional transport from basolateral to apical (BL-AP) side, respectively. The cells were then incubated at 37° and 5% CO2. At 30, 60, 90, 120 and 180 min time points, HBSS in the acceptor chambers was collected for analysis and replaced with equal volume of fresh warm HBSS. A fluorescent marker, lucifer yellow, was employed as a paracellular maker for checking the integrity of Caco-2 cell junction during the transport study. The apparent permeability coefficient, Papp (cm/ sec), was calculated using the following equation: Papp = (ΔQ/Δt)×(1/A×1/Cdonor), Where, DQ/Dt is the permeability rate (ng/s), A is the insert area of the transwell (cm2), Cdonor is the initial drug concentration added to the donor chamber (ng/ml).
Results and Discussion
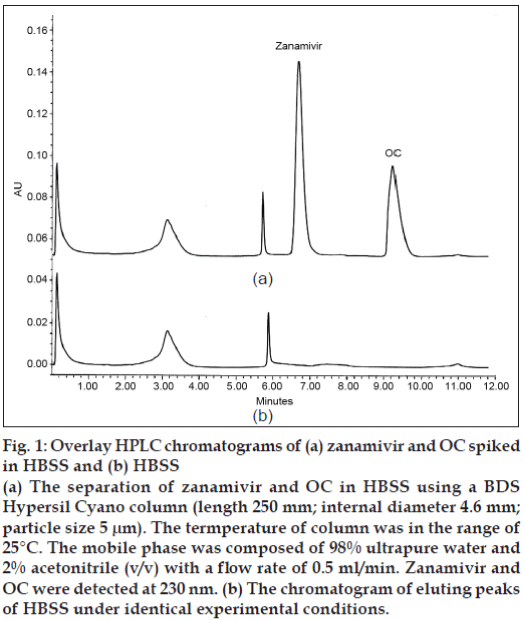
The reversed phase C18, C8 and C4 columns were initially employed in this study; however, components present in the HBSS co-eluted with zanamivir. The BDS Cyano column could solve these interference problems. By using the developed HPLC system, zanamivir and OC were eluted at 6.69 and 9.32 min, respectively (fig. 1). The standard curve of zanamivir solutions at a concentration range from 0.1-10 μg/ ml gave the coefficient of determination higher than 0.999 (fig. 2) indicating that the equation for the quantification of zanamivir concentration in HBSS was defined. The lower limit of quantification for the method was 0.1 μg/ml. The recovery of zanamivir at concentration of 0.1, 5 and 10 μg/ml was in the range of 99.76-105.08% (Table 1). The accuracy of the results proved that the mean test results were close to the true concentrations of analyte.
| Concentration(µg/ml) | Concentration determined(µg/ml) | % Recovery |
|---|---|---|
| 0.1 | 0.11±0.01 | 105.08 |
| 5 | 5.05±0.38 | 101.00 |
| 10 | 9.98±0.52 | 99.76 |
The values reported were the mean±SD of six determinations.
Table 1: Accuracy of zanamivir determination in hbss
Figure 1: Overlay HPLC chromatograms of (a) zanamivir and OC spiked in HBSS and (b) HBSS
(a) The separation of zanamivir and OC in HBSS using a BDS Hypersil Cyano column (length 250 mm; internal diameter 4.6 mm; particle size 5 μm). The termperature of column was in the range of
25°C. The mobile phase was composed of 98% ultrapure water and 2% acetonitrile (v/v) with a flow rate of 0.5 ml/min. Zanamivir and OC were detected at 230 nm. (b) The chromatogram of eluting peaks
of HBSS under identical experimental conditions.
The values of relative standard deviation (RSD) for within-day precision were between 5.32-10.32%, while those for between-day precision were lower than 14.33% (Table 2). The recovery of zanamivir at concentrations of 0.1, 5 and 10 μg/ml was in the range between 97.43 and 102.14% after three freezethaw cycles (Table 3). The short-term temperature stability for 36 h at room temperature was based on the duration that samples were maintained for the intended study. The recovery of the short-term temperature stability at concentrations of 0.1, 5 and 10 μg/ml was 95.65, 102.73 and 101.02%, respectively (Table 3). The results of stability test demonstrated that zanamivir was stable and no degradation of the drug was found due to the freeze-thaw process and zanamivir was stable in HBSS at room temperature for at least 36 h.
| Concentration (µg/ml) | Concentration determined(µg/ml) | RSD (%) |
|---|---|---|
| Within-day | ||
| 0.1 | 0.10±0.01 | 10.32 |
| 5 | 5.09±0.28 | 5.50 |
| 10 | 10.03±0.53 | 5.32 |
| Between-day | ||
| 0.1 | 0.11±0.02 | 14.33 |
| 5 | 5.12±0.49 | 9.57 |
| 10 | 10.03±0.65 | 6.52 |
Each value reported was from the analysis of six determinations
Table 2: Within-day and between-day variation of zanamivir determination
| Concentration (µg/ml) | Concentration | Concentration | Recovery as referredto basal level (%) |
|---|---|---|---|
| determined at basal | determined after | ||
| level (µg/ml) | stability test (µg/ml) | ||
| Freeze-thaw stability | |||
| (Three cycles) | 0.10±0.01 | 0.11±0.01 | 102.14 |
| 0.1 | 5.10±0.53 | 4.97±0.49 | 97.43 |
| 5 | 9.99±0.48 | 10.10±0.53 | 101.03 |
| 10 | |||
| Short-term temperature stability (room | |||
| temperature, 36 h) | |||
| 0.1 | 0.11±0.01 | 0.11±0.01 | 95.65 |
| 5 | 5.02±0.49 | 5.16±0.54 | 102.73 |
| 10 | 10.09±0.52 | 10.20±0.43 | 101.02 |
The values expressed were from the analysis of six determinations.
Table 3: Freeze-thaw stability and short-term temperature stability of zanamivir determination
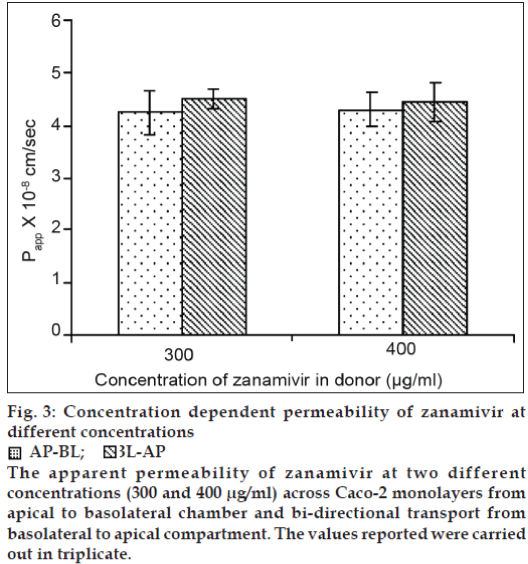
The passage of the Lucifer yellow was found to be lower than 0.5% per hour confirming the well differentiated tight junctions of the monolayers. Zanamivir at the concentrations of 300 and 400 μg/ ml had no significant cytotoxicity to Caco-2 cells compared to the control wells (P>0.05, two-tailed test, Student’s t-test). The TEER values for all conducted experiments were in the range between 702 and 856 ohm.cm2. The Lucifer yellow passage, a passive paracellular maker, was between 0.17-0.40% per hour. Using zanamivir concentrations of 300 and 400 μg/ ml, the ratio of Papp of AP-BL to BL-AP at each concentration was 0.94 and 0.97, respectively (fig. 3). There was no significant difference in the Papp values of zanamivir at two different concentrations for both AP-BL and BL-AP directions (P>0.05, two-tailed test, Student’s t-test), (fig. 3). The ratio of Papp of AP-BL to BL-AP closing to 1 indicated the transport direction had no effect on the permeability of zanamivir. Based on the results obtained and the similarity in the Papp values of zanamivir at two different concentrations for both AP-BL and BL-AP directions, it is likely that passive transport is involved in the permeability of zanamivir across the Caco-2 monolayers. To elucidate further the type of passive pathways (paracellular or transcellular), the permeability of zanamivir was performed in the HBSS omitting calcium. The Papp values of zanamivir increased 56.21 and 57.20 fold in AP-BL and BL-AP directions, respectively (Table 4). The Papp values of zanamivir in both AP-BL and BL-AP directions with the absence of calcium in the transport medium increased because the tight junctions between Caco-2 cells tend to open, and therefore enhancing the permeability of paracellularly transported compounds. The results suggested that zanamivir was predominantly transported across Caco-2 monolayers by passive diffusion via the paracellular pathway. The paracellular permeability in the GI tract primarily applies to compounds that have a molecular weight (MW) less than 180 Da [20]. Hence, the paracellular transport of zanamivir (MW~ 332) is limited due to the factor of its molecular size and this is one of the possible reasons causing low oral bioavailability of zanamivir. A simple and reliable HPLC method was developed and validated to study the mechanism of zanamivir absorption using Caco-2 cell model. Based on the permeability studies, zanamivir was extensively transported across Caco-2 monolayers via passive paracellular route. The findings of this investigation are helpful for the further study on the formulation development of zanamivir to increase its oral bioavailability.
| Transport medium | P (10-6 cm/sec) app |
|---|---|
| AP-BL | |
| HBSS | 0.043± 0.003 |
| HBSS without Ca2+ | 2.417±0.161 |
| BL-AP | |
| HBSS | 0.045±0.004 |
| HBSS without Ca2+ | 2.574±0.206 |
The values reported were carried out in triplicate.
Table 4: Permeability of zanamivir at concentration across caco-2 monolayers under different transport conditions
Figure 3: Concentration dependent permeability of zanamivir at different concentrations AP-BL;
AP-BL;  BL-AP
The apparent permeability of zanamivir at two different concentrations (300 and 400 μg/ml) across Caco-2 monolayers from apical to basolateral chamber and bi-directional transport from basolateral to apical compartment. The values reported were carried
out in triplicate.
BL-AP
The apparent permeability of zanamivir at two different concentrations (300 and 400 μg/ml) across Caco-2 monolayers from apical to basolateral chamber and bi-directional transport from basolateral to apical compartment. The values reported were carried
out in triplicate.
Acknowledgements
The authors thank the Thailand Research Fund for financial support (Grant No. TRG5080004).
References
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, Jong MD, et al. Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5, Current concepts: Avian influenza A (H5N1)infections in Humans. N Engl J Med 2005;353:1374-85.
- Moscona A. Oseltamivir resistance-disabling our influenza defenses. N Engl J Med 2005;353:2633-6.
- Govorkova EA, Leneva IA, Goloubeva OG, Bush K, Webster RG. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamiviragainst H5N1, H9N2, and other avian influenza viruses. AntimicrobAgents Chemother 2001;45:2723-32.
- Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al. Resistant influenza A viruses in children treated with oseltamivir: Descriptive study. Lancet 2004;364:759-65.
- Wei DQ, Du QS, Sun H, Chou KC. Insights from modeling the 3D structure of H5N1 influenza virus neuraminidase and its binding interactions with ligands. BiochemBiophys Res Comm 2006;344:1048-55.
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KY, et al. Avian flu: Isolation of drug-resistant H5N1 virus. Nature 2005;438:754.
- Escuret V, Frobert E, Bouscambert-Duchamp M, Sabatier M, Grog I, Valette M, et al. Detection of human influenza A (H1N1) and B strains with reduced sensitivity to neuraminidase inhibitors. J ClinVirol2008;41:25-8.
- Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med 2005;353:1363-73.
- McKimm-Breschkin JL, Sahasrabudhe A, Blick TJ, McDonald M, Colman PM, Hart GL, et al. Mutations in a conserved residue in the influenza virus neuraminidase active site decreases sensitivity to Neu5Ac2en-derived inhibitors. J Virol 1998;72:2456-62.
- Muramoto Y, Le TQ, Phuong LS, Nguyen T, Nguyen TH, Sakai-Tagawa Y, et al. Molecular characterization of the hemagglutinin and neuraminidase genes of H5N1 influenza A viruses isolated from poultry in Vietnam from 2004 to 2005. J Vet Med Sci 2006;68:527-31.
- Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005;353:2667-72.
- Gupta RK, Nguyen-Van-Tam JS, Jong MD, Hien TT, Farrar J. Oseltamivir resistance in influenza A (H5N1) infection. N Engl J Med 2006;354:1423-4.
- Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivirafter intravenous, oral, inhaled or intranasal administration to healthy volunteers. ClinPharmacokinet 1999;36:1-11.
- McKimm-Breschkin JL. Resistance of influenza viruses to neuraminidase inhibitors-A review. Antiviral Res 2000;47:1-17.
- Liang-Shang G, Poe-Hirr H, Pritchard JF, Thakker D. Mechanism of intestinal absorption of ranitidine and ondansetron: Transport across Caco-2 cell monolayers. Pharm Res 1993;10:1722-5.
- Lennernas H. Human intestinal permeability. J Pharm Sci 1998;87:403-10.
- Ooie T, Terasaki T, Suzuki H, Sugiyama Y. Quantitative brain microdialysis study on the mechanism of quinolones distribution in the central nervous system. Drug MetabDispos 1997;25:784-9.
- Chandler M, Bamford MJ, Conroy R, Lamont B, Patel B, Patel VK, et al. Synthesis of the potent influenza neuraminidase inhibitor 4-guanidino Neu5Ac2en. X-Ray molecular structure of 5-acetamido-4-amido-2,6-anhydro-3-4,5-trideoxy-D-erythro-L-gluco-nononic acid. J ChemSoc Perkin Trans 1995;1:1173-80.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Kerns EH, Di L. Drug-like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization. Burlington, MA, USA: Academic Press; 2008.