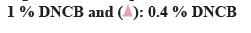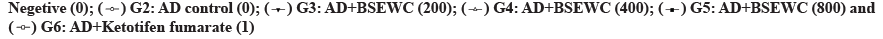- *Corresponding Author:
- Kyung Seuk Song
Korea Conformity Laboratories, Gaetbeol-ro 145 beon-gil, Yeonsu-gu, Incheon 21999, Korea
E-mail: songks@kcl.re.kr
| Date of Received | 17 May 2020 |
| Date of Revision | 13 February 2022 |
| Date of Acceptance | 14 June 2022 |
| Indian J Pharm Sci 2022;84(3):750-761 |
Abstract
Bamboo salt and egg white-chalcanthite mixture has been prescribed for relieving the inflammation of atopic dermatitis in Hamyang, Gyeongsangnam-do, Korea. This study was conducted to demonstrate the effect of atopic dermatitis on cell and atopic animal models using a combination of bamboo salt with egg white-chalcanthite, which have been used as folk remedies for inflammatory conditions. Based on the in vitro experiment, the 1:20 mixture of egg white-chalcanthite and bamboo salt was shown to inhibit the release of beta-hexosaminidase and histamine in rat basophilic leukemia cell line RBL-2H3 by sensitizing immunoglobulin E and inducing allergic responses by dinitrophenyl as well as the expression of inflammatory cytokines and inflammatory mediators in a dose-dependent manner in lipopolysaccharide-treated mouse macrophage cell line RAW 264.7 cells. Furthermore, egg white-chalcanthite with bamboo salt and its combination at different mixing ratios showed a decrease in inflammatory mediators. Dinitrochlorbenzene was applied to NC/Nga mice for 6 w to induce the atopic in vivo model. After 4 w administration of the test substance, the dermatitis decreased visually and dermal hyperplasia/inflammation, mast cell/eosinophils counts and degree of nerve fiber infiltration were decreased according to histopathological examination. In addition, the secretion rate of interferon gamma in high-dose treated group was lower than that in atopic dermatitis control group. These results show that bamboo salt with egg white-chalcanthite effectively relieves skin inflammation in atopic dermatitis and it might be a promising mineral agent for relieving the symptoms of atopic dermatitis.
Keywords
Atopic dermatitis, bamboo salt, egg white chalcanthite, inflammation
Atopic Dermatitis (AD) is a multifactorial immune disorder that causes serious symptoms including severe skin irritation. Skin barrier anomalies and overactive immune systems secrete modulators to the skin surface, causing inflammation and skin irritation[1]. The rash on the skin of patients with AD is visible only near the dermis. Even though the skin looks normal, there may still be inflammation near the dermal layer of the skin. Additionally, scratching the itchy skin will peel off the stratum corneum of the skin surface, causing bacteria, viruses and allergens to enter the body followed by secretion of immunological elements close to the skin surface causing irritation to the skin and mucous membranes, accompanied by redness and itching[2].
AD is one of the several chronic recurrent skin diseases that require continuous treatment. In general, antihistamines and atopic treatments can alleviate the tingling sensation in the skin but fail to serve as an effective long-term remedy[3]. Studies investigating the effect of suppressing atopy and improving atopic symptoms using natural products and oriental medicine have been carried out in Korea with a goal to develop novel and specific drugs[4-7]. Furthermore, it has been reported that a healthy balance between natural moisturizing factors and dryness of the skin may be critical in maintaining skin health[8]. Therefore, studies testing the efficacy of certain nutrients or dietary materials containing nutrients related to dryness of skin have been actively conducted. In the past, health supplements containing food ingredients with medicinal value were developed and marketed. However, in recent times, functional foods or cosmetics made from food extracts are receiving promising medical attention[9,10]. Despite several studies in this field, no breakthrough treatment for atopy has yet been devised.
The mixture of bamboo salt and egg white-chalcanthite has been prescribed for relieving the inflamed condition in AD in Hamyang-eup, Hamyang-gun and Gyeongsangnam-do, Korea in past decades. In this study, we investigated the efficacy of bamboo salt and egg white-chalcanthite composition, which was used for prevention and treatment of skin diseases by folk medicine. After confirming the effect of the substance in vitro, the efficacy of dermatitis treatment was evaluated to confirm the possibility of development of the substance as a medication for AD in an animal model.
Materials and Methods
Preparation of the test substance:
The chalcanthite, natural mineral containing Copper sulfate pentahydrate (CuSO4.5H2O) was heated at 140° for 4 h to be completely dehydrated, cooled at room temperature and then powdered into a mortar (fig. 1A-fig. 1C). Chalcanthite powder and egg white was mixed at a ratio of 1:0.7, cooled as shown in fig. 1D and then powdered again with mortar. The 9th step molten bamboo salt was produced by the following procedure; sun-dried salt, obtained from the western seacoast of Korea was added into the bamboo (70~100 mm φ) and capped with red clay; bamboo with sun-dried salt was piled up one by one into brazier and burned at 800° with pine wood; remaining salt pillars were crushed; salt was put into new bamboo slice, sealed with red clay, burned and crushed 8 times, similar to that in first step; in the final 9th step, melting, pine resin was added to the salt and then heated up to 1400°; liquefied salt sample was cooled at room temperature for 12 h and finely pulverized as shown in fig. 1E. The samples, egg white-chalcanthite, bamboo salt and their combination (fig. 1F), were uniformly dissolved in sterilized distilled water at a concentration of 100 mg/ml and the materials were filtered with 0.2 μm pore membrane (Advantec®, 25CS020AS). The test solution was prepared immediately prior to treatment.
Standard compound analysis:
Copper was selected as the major component of the bamboo salt with egg white-chalcanthite because it is a suitable mineral for quality control. Copper was analysed by Atomic Absorption Spectroscopy (AAS), AA280FS, varian, USA system. Analysis was performed using air/acetylene flame type Copper Hollow Cathode (Cu HC) lamp (varian, USA) under 13.98 l/min gas flow, 1.78 l/min acetylene, 324.8 nm absorbance wavelength and 5 mA current. The standard material was used International Comparison Program (ICP) quality control standard #3 (AccuStandard, Inc.). Egg whitechalcanthite, bamboo salt, egg white-chalcanthite and bamboo salt mixture (1:20) was analysed respectively. Each test substance was prepared with 1 % (0.3 g/30 ml distilled water, filtered through a 0.2 μm syringe filter and then injected with nitric acid to a concentration of 2 % (v/v). The 9th molten bamboo salt was not diluted because egg white-chalcanthite and mixture was diluted 100 and 2000 times, respectively, with 2 % nitric acid.
Cell culture:
Rat Basophilic Leukemia (RBL-2H3) cell line and a mouse macrophage cell line (RAW 264.7), were purchased from the Korean Cell Line Bank (Seoul, Korea). Dulbecco's Modified Eagle's Medium (DMEM) containing 10 % heat-inactivated Fetal Bovine Serum (FBS), 2 mm L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin were used to maintain the cell culture. Both cell lines were incubated under 5 % Carbon dioxide (CO2), at 37°.
Cell viability assay:
A preliminary test was conducted by treating the two cell lines with 200, 40, 8, 1.6 and 0.32 μg/ml concentration of Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc., kamimashiki gun, Kumamoto, Japan, lot no. KL709). Cells were seeded at 2×104 cells/ well in a 96-well plate and incubated in a CO2 incubator at 37° for 24 h. After growth medium was removed, the cells were treated with the test solution (200, 40, 8, 1.6, 0.32 μg/ml) and cultured for 24 h. CCK-8 solution (10 μl) was added to the cell culture medium (100 μl). After incubation for 2 h in a CO2 incubator, absorbance was measured at 450 nm using a microplate reader (Molecular devices, SoftMax Pro5, USA). Based on the measured optical density value, the cell viability was calculated and the final Lethal Concentration 50 (LC50) was determined.
Beta (β)-hexosaminidase assay:
Cells suspended in culture medium were plated at 4×105 cells/well in a 6-well plate and incubated in a 37°, CO2 incubator for 8 h. After removing the existing medium, anti-Dinitrophenyl (DNP) Immunoglobulin E (IgE) diluted to 0.1 μg/ml was added to the serum-free medium for sensitization. Test solutions of egg white-chalcanthite, bamboo salt, 100 mg/ml ketotifen fumarate and mixture of egg white-chalcanthite and bamboo salt (1:10, 1:15, 1:20) were treated overnight. After washing three times with Tyrode buffer (135 mM Sodium chloride (NaCl), 5 mM Potassium chloride (KCl), 20 mM 4-(2-Hydroxyethyl)-1- Piperazineethanesulfonic Acid (HEPES), 5.6 mM glucose, 1.8 mM Calcium chloride (CaCl2), 1 mM Magnesium chloride (MgCl2), 0.05 % Bovine Serum Albumin (BSA), pH 7.4), 0.01 μg/ml DNPBSA (antigen) was added and incubated for 1 h. The supernatant was collected and distilled water was added for harvesting cells attached to the well plate. Each sample (15 μl) was dispensed in a 96- well plate, followed by the addition and incubation of 60 μl of 1 mM para (p)-nitrophenyl-N-acetyl- β-D-glucosaminide for 1 h. Carbonate buffer (150 μl, 0.05 M, pH 10) was added and the absorbance at 405 nm was measured. All trials were conducted in triplicate.
Histamine assay:
Cells were treated similar to when measuring β-hexosaminidase. After incubation with the test substance, the supernatant was collected and the cells were collected and washed in Phosphate Buffered Saline (PBS). Cells and supernatants were tested using a histamine Enzyme-Linked Immunoassay (ELISA) kit (Oxford Biomedical Research, Oxford, MI, USA) following manufacturer’s guidelines. All trials were conducted in triplicate.
Measurement of inflammatory mediators and inflammatory cytokines:
RAW 264.7 cells were seeded at 4×105 cells/well in a 6-well plate and cultured in a 37° CO2 incubator for 24 h. Cells were incubated with Lipopolysaccharide (LPS) (1 μl/ml) for 1 h and cultured for 24 h with the test substance. Cell supernatants were collected and Interleukin (IL)-1β, IL-6, Tumour Necrosis Factor alpha (TNF-α) and Prostaglandin E2 (PGE2) were measured by ELISA, following manufacturer’s guidelines (IL-1β, IL-6, TNF-α ELISA kit; R&D Systems, Minneapolis, MN, USA, PGE2 ELISA kit; Abcam, Cambridge, MA, USA).
Animal experiment:
Animals: The animal study was approved by the animal care and use committee at the Korea conformity laboratories (IA16-00036). NC/NgaTndCrlj mice were purchased from Orient Bio (Charles River Japan, 322, Galmacho, Jungwon-gu, Sungnam, Korea). On the day of receipt, a microbiological test report was received and animal appearances were carefully examined. Animals were maintained in a laboratory facility at 22°±3° and a relative humidity of 50 %±10 % under a 12:12 h light and dark cycle. Intensity of illumination was LX 300. Noise level was 60 dB or less. During the experimental period, the mice were fed a rodent diet (Teklad Certified Irradiated Global 18 % protein rodent diet, Harlan Co. Ltd., WI, USA) supplied by DooYeol Biotech Co., Ltd. (107 Seongbo plaza, 91, Baumoe-ro, Seocho-gu, Seoul, Korea). All animals were provided with tap water purified by a reverse osmosis filtering system ad libitum. All animals were acclimated for 6 d after acquisition.
Induction of AD and grouping:
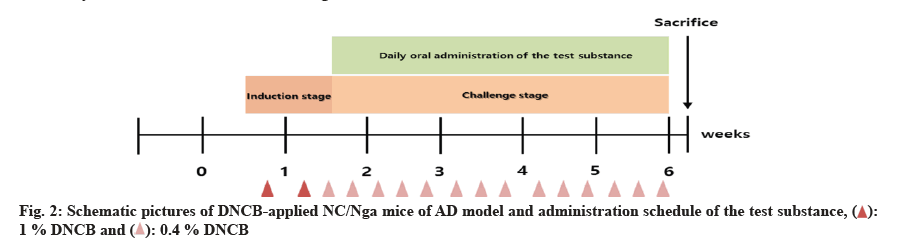
For the induction of AD, the hair on the back of the animal was shaved using an electric razor and hair removal cream on the 6th d after arrival in the animal facility. 2,4-Dinitrochlorobenzene (DNCB) (200 μl, 1 %) was applied to the skin for primary sensitization and 2 d later, 2nd induction was induced was induced by the same method. 4 d after the second sensitization, 150 μl of 0.4 % DNCB was applied to the epidermal skin to induce dermatitis (challenge stage) three times a week for 6 w. Sensory evaluation was conducted prior to initiation of administration and grouping was performed by randomization based on the scores. Fifty mice with AD were divided into 5 groups of 10 mice. Test groups were administered with 800 (high-dose), 400 (mid-dose), 200 mg/kg body weight (low-dose) concentrations and the effects were compared to those in the AD control and positive groups. After induction of AD, the test solution was administered by oral gavage once a day, 7 d/w for 4 w, as shown in fig. 2.
Clinical observation and weight measurement:
The general symptoms were observed immediately after drug administration, once a day. Individual records were maintained for each animal including the mortality, type, date and grade of clinical signs. Individual animal weight was recorded at acquisition, grouping, before administration, necropsy once a week during the study.
Sensory evaluations of skin lesions:
This evaluation method was used to compare scores by the modified SCORing Atopic Dermatitis (SCORAD) scoring system, which is a clinical visual evaluation method generally used in AD. According to the index of dermatitis scoring method, the degree of skin lesions was divided into three categories scaling/dryness, edema and excoriation/erosion. Scores were given between 0 and 9, with 0 (no change), 1 (weak change), 2 (moderate change) and 3 (severe change) for each item. Sensory evaluation was conducted on the 1st d of administration and once a week before necropsy.
Hematological test and serum IgE measurement:
On the day of autopsy, the animals were anesthetized with isoflurane and blood samples were drawn from the abdominal aorta and collected in Dipotassium Ethylenediaminetetraacetic acid (EDTA-K2) tube (Becton Dickinson, San Jose, CA, USA). The blood was analysed using a haematology analyser (ADVIA 2120, SIEMENS, Germany). White Blood Cell (WBC) count, neutrophil, eosinophil, basophil, lymphocyte, monocyte and large unstained cell counts, and the percentages of neutrophils, eosinophils, basophils and lymphocytes were analysed. The amount of IgE in blood serum was measured using an IgE mouse ELISA kit (Abcam, Cambridge, MA, USA. Lot No. GR3221697-1).
Histopathological examination:
Histopathological examination of skin tissue was performed after autopsy. Hematoxylin and Eosin (HE) staining was performed to confirm the lesion in skin tissue and mast cell and eosinophil infiltration was confirmed by toluidine-blue and Congo red staining. In HE staining, the lesion was classified as dermal hyperplasia, dermal inflammation, epidermal erosion/ ulcer, hyperkeratosis and dermal fibrosis. The score was given from 0 to 4 as follows 0 is normal, 1 is minimal, 2 is mild, 3 is moderate and 4 is severe. In toluidine blue staining, the score was assigned, depending on the distribution of mast cells as follows: 0: 0 to 10, 1: 10 to 30, 2: 30 to 70, 3: >70. In Congo red staining, the score was given depending on the distribution of eosinophils as follows: 0: 0, 1: <10, 2: 10-30, 3: 30-70, 4: >70. Mast cells and eosinophils were scored under 400× magnification.
Immunohistochemistry (IHC) of Protein Gene Product (PGP) 9.5:
Sections (5 μm) were cut from all paraffin blocks and mounted on glass slides. The sections were deparaffinized by three immersions in xylene and then hydrated by descending concentrations of ethanol (100 %, 90 % and 70 %) to PBS. After 1 h immersion in hydrogen peroxide solution, the slides were then blocked in goat serum and incubated overnight with mouse PGP 9.5 antibody (Invitrogen, Waltham, MA, USA; 1:100). After washing, Dako Real Envision detection system (K5007, Dako, Santa Clara, CA, USA) was used to allow for quantitative detection neuroendocrine marker PGP 9.5 respectively. The samples from each section were evaluated at 40× magnification, using a microscope (Olympus Provis AX-70; Olympus USA) equipped with a camera (Olympus U-MAD 2, Olympus, Japan). The images were captured using the program MagnaFire 2.1B (Olympus).
Measurement of cytokine secretion in splenocytes:
Spleens were aseptically harvested. Using the plunger end of the syringe, mesh the spleen through the cell strainer into a falcon tube. Strainer was rinsed several times with serum free Roswell Park Memorial Institute (RPMI) 1640 medium. Cells were centrifuged at 300 g for 10 min at room temperature. The supernatant was removed and cell pellet was suspended in Ammonium- Chloride-Potassium (ACK) lysing buffer (A10492, Invitrogen, Waltham, MA, USA) and taped for 15 min to remove the red blood cells. After washing with serum free media, the cells were suspended in a RPMI 1640 medium supplemented with penicillin 100 U/ml and streptomycin 50 μg/ml, 2-mercaptoethanol, Non-Essential Amino Acid (NEAA) and FBS, 1 μg/ well anti-CD3 mAb (eBioscience, San Diego, CA, USA) and 1 μg/well anti-CD28 mAb (ebioscience, San Diego, CA, USA) were added and incubated for 48 h. After incubation, the cytokine released into the culture medium was assayed using the Mouse IL-4 Quantikine (R&D systems, Minneapolis, MN, USA, Lot No. P161144) and the mouse Interferon‐gamma (IFN‐γ) Quantikine ELISA kits (R&D systems, Lot No. P161140).
Statistical analysis:
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 12.0 K program (SPSS, Chicago, IL, USA). Numerical data was calculated by mean±standard deviations. Differences in means were considered statistically significant when p<0.05. Statistical significance was represented by percentage. The statistical differences between vehicle and DNP control (in vitro)/vehicle and AD control (in vivo) were examined using student’s t-test. The statistical differences among test groups except vehicle control were examined by standard one-way Analysis of Variance (ANOVA). If the difference was statistically significant, the data were analyzed by parametric multiple comparison to evaluate the DNP control. If the equal variance was admitted, Duncan’s test was used and if the equal variance was not admitted, Dunnett’s test was applied. In the hematological analysis, one animal each from G2: AD control (0), G3: AD+BSEWC (200), G5: AD+BSEWC (800) and G6: AD+Ketotifen fumarate (1), was excluded from the statistical analysis due to difficulty obtaining blood sample. Seven individual samples were used for IgE and cytokine analysis owing to lack of sample volume.
Results and Discussion
Copper is classified as a nutrient in functional health foods, and is required for the transport and utilization of iron and protection cells from harmful oxygen. Since copper is an easy-to-analyse mineral, it was selected as an indicator for quality control of the test solution. The content of copper in egg white-chalcanthite, bamboo salt and mixture of egg white-chalcanthite and bamboo salt (1:20) was 19.48 %, 0 % and 0.98 %, respectively.
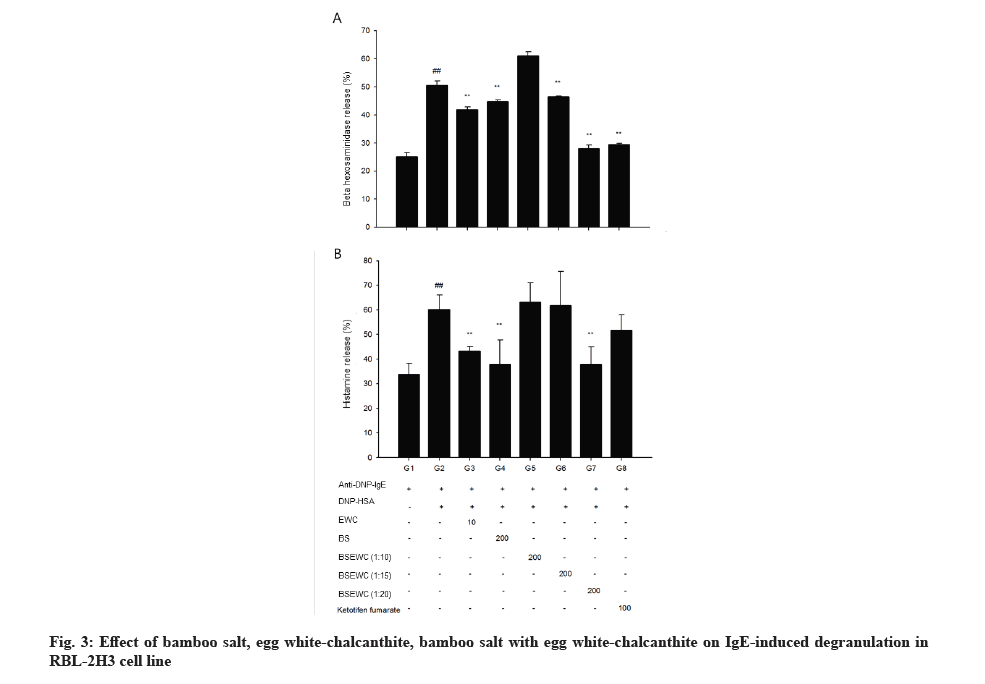
β-hexosaminidase secretion was measured as an indicator for the inhibitory effect of degranulation, which is an index of antigen-antibody reaction. Comparative analysis of β-hexosaminidase released by cells showed that significantly elevated amounts (p<0.01) in the DNP control compared to the negative control. The amount of β-hexosaminidase released in egg white-chalcanthite, bamboo salt, 1:15 and 1:20 mixture-treated groups was 42 %, 45 %, 46 % and 28 %, respectively. This reduction was statistically significant when compared to that of DNP control (p<0.01). The positive control group showed a decrease of 29 % in degranulation, which was similar to that of the 1:20 mixture treated group (p<0.01) (fig. 3A).
The amount of histamine was measured as an indicator of the inhibitory effect of degranulation which is an index of the antigen-antibody reaction. The amount of histamine within the cell as well as that secreted by the cells was compared. DNP control showed 60 % histamine release and negative control showed 34 % release from the cells (p<0.01). The egg white-chalcanthite, bamboo salt, 200 μg/ml 1:20 mixture treated groups showed 28 %, 37 % and 37 % histamine release, respectively. It was a statistically significant decrease when compared to that of DNP control (p<0.01) (fig. 3B).
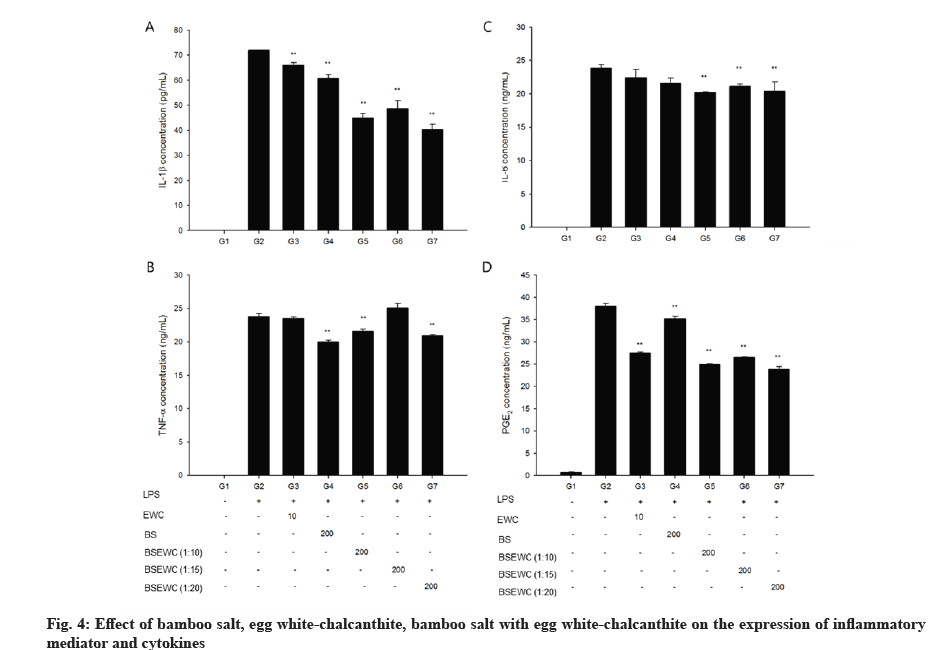
Significantly elevated levels of IL-1β were observed in the LPS control group compared to the vehicle control group. However, incubation with 10 μg/ml egg white-chalcanthite; 200 μg/ml bamboo salt; 1:10, 1:15 and 1:20 egg white-chalcanthite and bamboo salt mixture-treated groups showed reduced levels of IL-1β, suggesting a reduction in inflammation (p<0.01) (fig. 4A). Similarly, elevated expression of IL-6 was in the LPS control group was significantly attenuated in 200 μg/ml 1:10, 1:15 and 1:20 egg white-chalcanthite and bamboo salt mixture-treated groups compared to the LPS control group (p<0.01) (fig. 4B). The expression of TNF-α, although increased in the LPS control group compared to the vehicle control group, was significantly decreased in 200 μg/ml bamboo salt, 1:10, 1:20 egg white-chalcanthite and bamboo salt mixture-treated groups compared to the LPS control group (p<0.01) (fig. 4C). The expression of PGE2 was increased in the LPS control group compared to the vehicle control group, but was reduced in all test substance-treated groups compared to LPS control group (p<0.01) (fig. 4D). In particular, 1:20 egg white-chalcanthite and bamboo salt mixture effectively reduced the release of inflammatory cytokines and mediators in all test items.
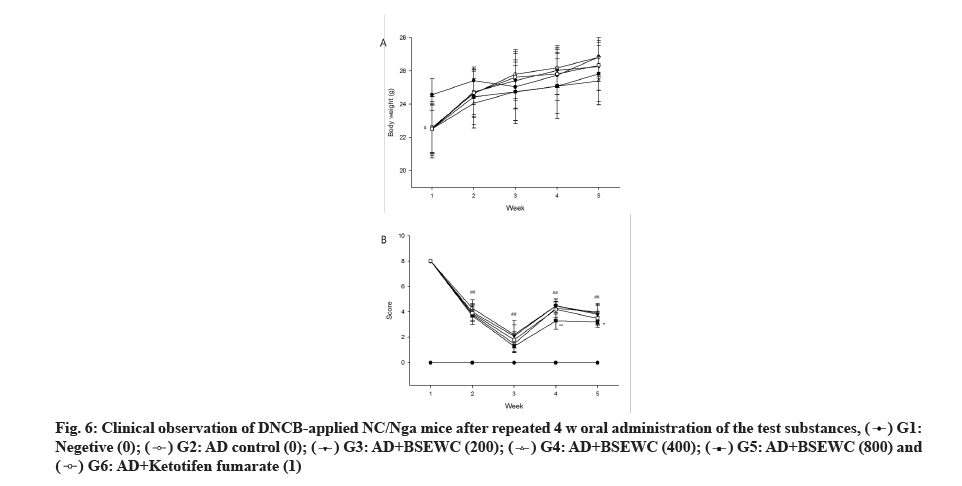
No death or other abnormalities were observed except for AD in the AD-induced animal group during the experimental period. Weight-measurement results indicated a reduction in 1 w body weight of ADinduced group compared to the normal control group (p<0.05). However, this change was temporary and a normal weight gain was observed thereafter (fig. 5A). Skin sensory evaluation results indicated a significant reduction in the AD-induced group compared to the ADinduced control group at 3 and 4 w after administration of 800 mg/kg/d (p<0.01 or p<0.05) (fig. 5B).
Hematological tests showed that WBC in 800 mg/kg/d treated group and monocyte levels in 800 mg/kg/d and ketotifen fumarate-treated groups were statistically higher than AD induced excipient control group (p<0.05 or p<0.01). No statistically significant changes were observed in other test items compared to AD control group (fig. 6A). The blood IgE concentration did not show statistically significant changes in the test substance administration group, but the blood IgE concentration in the test substance-treated group and the positive control substance were decreased compared to that in the AD-induced control group (fig. 7A). IL-4 level was significantly decreased in splenocytes from the group treated with 400 mg/kg bamboo salt with egg white-chalcanthite (p<0.01) (fig. 7B). In the 800 mg/ kg and ketotifen fumarate-treated groups, IFN-γ level was reduced (fig. 7C) and the IL-4/IFN-γ ratio was significantly increased compared to in the AD control group (fig. 7D).
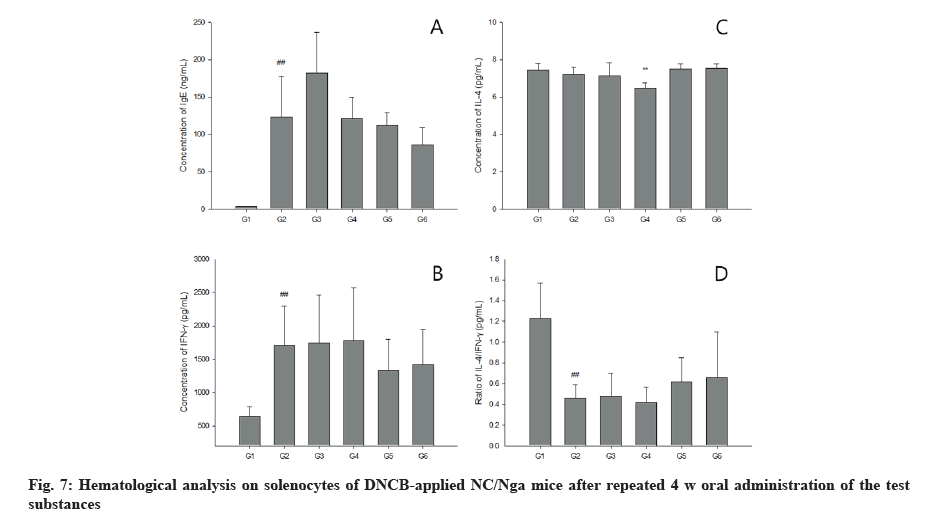
The vehicle control did not show any abnormality in terms of lesions observed in the skin tissue and all items were scored as 0. AD was observed in AD control mice with the highest scores in dermal hyperplasia and inflammation confirming robust induction of AD. In the 200, 400 and 800 mg/kg 1:20 mixture of egg whitechalcanthite and bamboo salt-treated group, the score of dermal hyperplasia and inflammation decreased in a dose-dependent manner. Particularly in the 800 mg/kg treated group, the scores of hyperkeratosis and dermal fibrosis decreased simultaneously. The AD control group got the highest score in the sum of each parameter and the score was reduced in the test substance-treated group in a dose-dependent manner. However, there was no significant difference in epidermal erosion/ulcer score among the groups. The positive control group showed a similar score to the 800 mg/kg treated-group.
The AD control group exhibited the highest mast cell counts while the vehicle group had a score of 0. In the 200, 400 and 800 mg/kg 1:20 mixture of egg whitechalcanthite and bamboo salt-treated groups, the score decreased in a dose-dependent manner. The positive control group got the lowest score. The vehicle control group was awarded a score of 0 in terms of eosinophil counts. The scores of 200, 400, and 800 mg/kg 1:20 mixture of egg white-chalcanthite and bamboo salttreated groups were reduced in a dose-dependent manner because the AD control group did not show the highest score. The positive control group received the same score as the 800 mg/kg-treated group (fig. 8).
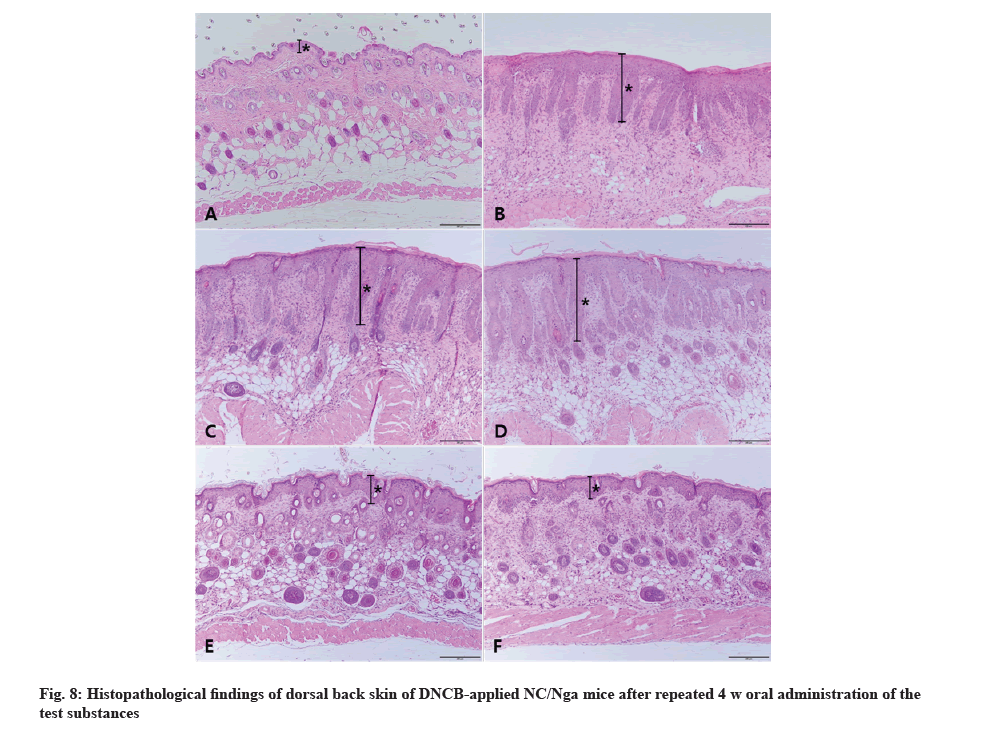
PGP 9.5 was immunohistochemically stained to confirm the degree of nerve fiber infiltration. IHC results showed high nerve fiber infiltration in AD control group skin tissue whereas infiltration was barely observed in the vehicle control group. The degree of nerve fiber infiltration in bamboo salt with egg white-chalcanthitetreated group was lowered in a dose-dependent manner. The positive control group showed similar degree of invasion as the 800 mg/kg bamboo salt with egg whitechalcanthite- treated group (Fig. 9).
Based on the above results, it was found that when the test substance, bamboo salt with egg white-chalcanthite, was orally administered to the AD model for 4 w repeatedly, the dermatitis decreased visually, dermal hyperplasia/inflammation, mast cell/eosinophils counts and degree of nerve fiber infiltration were decreased according to histopathological examination. In addition, the secretion rate of IFN-γ in high-dose treated group was lower than that in AD control group. And, bamboo salt and egg white-chalcanthite help to relieve symptoms of AD in vitro as well. We believe that the results of this study will encourage further research on the anti-inflammatory effect of the combination of egg white-chalcanthite and bamboo salt to develop novel therapeutic agents to treat widespread dermatological conditions such as AD.
Egg white-chalcanthite and bamboo salt mixture is a medicinal product that has been traditionally used to treat AD in Hamyang, Korea. One of the ingredients, chalcanthite is a mineral medicinal herb that contains naturally occurring copper vitriol, and is described as “stone gall” in Shennong's Materia Medica, Shen nong ben cao jing. The major components of the chalcanthite are Cupric oxide, carbonate and water whereas zinc, gallium, titanium, sodium, calcium, iron, aluminum, magnesium, silicon, chromium and nickel are minor constituents[11,12]. The efficacy of chalcanthite as a single medicinal product is presumed to be lowered in toxicity and higher in absorption effect when administered after processing with egg white. Combination with egg white can prevent liver dysfunction as well as disorders of the basal ganglia of brain and kidneys, when copper is consumed for prolonged periods of time[13]. Despite its diverse medicinal applications, research studies on chalcanthite have not been conducted while studies on mineral medicine have been quite limited. This is due to a general lack of concern about the toxicity of mineral medicines and an overall lack of systematic pharmacological analysis. Mineral medicines have been passed down through thousands of years of history alongside botanical and animal derived medicines[14,15]. If the active component and efficacy of medicinal minerals are identified in a scientific way, this may contribute to developing more effective novel drug medicines.
The other ingredient, bamboo salt, is a traditionally used medicinal ingredient, described as salt in various pamphlets such as in the traditional book, Donguibogam. However, it is not specifically referred to as bamboo salt. It is believed that though historically it has existed in multiple forms, the name bamboo salt itself was used since 1981[16]. Currently, people believe that one of the causes of hypertension is excessive sodium intake and as a result, salt consumption as a medicine may be viewed with some concern. Minerals are essential for human activity and are often obtained in adequate amounts through salt consumption[17]. The most commercially produced salt is NaCl. However, it is known that CaCl2, magnesium, potassium, selenium and germanium are relatively high in natural sun dried-salt, salt from vegetable (Salicornia europaea L.) or bamboo salt[18]. In particular, manufacturers frequently claim bamboo salt to be devoid of all the bad ingredients of refined salt that contains a significant amount of minerals and trace elements such as magnesium and calcium, which are beneficial to the human body[19]. Devoid of toxicity and with increased concentration of minerals, bamboo salt may be of significant therapeutic value. There are scientific explanations for the relief from inflammation in mineral AD as follows, Yoou et al.[20] reported that skin inflammation releaved by oral administration of bamboo salt in atopic dermititis mouse model. Kim et al.[21] reported that magnesium-rich marine minerals could be used as an adjunctive therapy, and Yoon et al.[22] reported that selenium-rich hot spring water has an anti-inflammatory and antimicrobial effect in chronic bacterial prostatitis rat. Further studies are required to elucidate the relationship between the mineral content in bamboo salt and dermatitis.
Recent research has shown that bamboo salt with egg white-chalcanthite has tremendous potential as a therapeutic agent in multiple diseases. It is reported that bamboo salt with egg white-chalcanthite can be developed and used as an effective therapeutic agent having anticancer effect in lung cancer[23], therapeutic effect in arthritis[23-25], and induction of apoptotic cell death in vitro[26]. Since egg white-chalcanthite and bamboo salt mixture exhibits various efficacy, toxicity test results using various routes of administration are essential for widespread use in clinical practice. Singledose, repeated-dose, sensitization, skin irritation, and genotoxicity tests are necessary and the side effects of administration should be closely examined.
Acknowledgements:
This work was supported by the technology development program (No. S2424212) funded by the Ministry of SMEs and Startups (MSS, Korea). Somin Lee, Hyeon Yeol Ryu and Eun A Choi conceived the idea and designed the experiments. Somin Lee, Hyeon Yeol Ryu, Su Jin Kang and Hye Jin Kim performed the experiments. Eun A Choi, Hong Geun Kim, Hyeong Ho Seo, Ji Young Choi contributed materials and reagents. Somin Lee analysed the data and wrote the manuscript. Kyung Seuk Song and Chanhee Chea reviewed the manuscript.
Conflict of interest:
The authors have no conflicts of interest to declare.
References
- Saini S, Pansare M. New insights and treatments in atopic dermatitis. Immunol Allergy Clin North Am 2021;41(4):653-65.
[Crossref] [Google Scholar] [PubMed]
- Pavlis J, Yosipovitch G. Management of itch in atopic dermatitis. Am J Clin Dermatol 2018;19(3):319-32.
[Crossref] [Google Scholar] [PubMed]
- Chu M, Tsang MS, He R, Lam CW, Quan ZB, Wong CK. The active compounds and therapeutic mechanisms of pentaherbs formula for oral and topical treatment of atopic dermatitis based on network pharmacology. Plants 2020;9(9):1166.
[Crossref] [Google Scholar] [PubMed]
- Ryu DH, Oh SR, Jung TS, Ryu DS. The effect of Angelica gigas Nakai extract and Bacillus polyfermenticus KJS-2 on atopic dermatitis induced by DNCB in mice. J Korea Med 2017;38(3):30-42.
- Seo S, Lee H, Choi M. The anti-inflammatory actions and dermal bioactive effects of Coreopsis lanceolata extracts. J Kor Soc Cosmetol 2018;24(3):472-81.
- Yang B, Kim S, Choi CH, Jeoung HW, Kim H. Effects of Scutellaria baicalensis extract on skin lesion of contact dermatitis induced by DNFB in Mice. J Physiol Pathol Kor Med 2017;31(1):59-64.
- Kim SH, Seong GS, Choung SY. Fermented Morinda citrifolia (Noni) alleviates DNCB-induced atopic dermatitis in NC/Nga mice through modulating immune balance and skin barrier function. Nutrients 2020;12(1):249.
[Crossref] [Google Scholar] [PubMed]
- Yosipovitch G, Misery L, Proksch E, Metz M, Ständer S, Schmelz M. Skin barrier damage and itch: Review of mechanisms, topical management and future directions. Acta Derm Venereol 2019;99(13):1201-9.
[Crossref] [Google Scholar] [PubMed]
- Wollenberg A, Fölster‐Holst R, Saint Aroman M, Sampogna F, Vestergaard C. Effects of a protein‐free oat plantlet extract on microinflammation and skin barrier function in atopic dermatitis patients. J Eur Acad Dermatol Venereol 2018;32:1-15.
[Crossref] [Google Scholar] [PubMed]
- Siti-Arffah K, Ridzuan PM, Norliany MY. Effect of herbal medicine on atopic dermatitis: A review. Ann Roman Soc Cell Biol 2021:4944-9.
- Chalcanthite, http://www.mindat.org/min-959.html
- http://rruff.geo.arizona.edu/doclib/hom/chalcanthite.pdf
- Choi EA, Lee JH, Youn DH, Yoo HS. Study on oral administration of egg white combined chalcanthite and bamboo-salt with egg white combined chalcanthite. J Physiol Pathol Kor Med 2012;26(2):189-98.
- Kim SO, Park ME. Standardization studies for the oriental mineral medicine. Econ Environ Geol 2015;48(3):187-97.
- Bak J, Kim D. Mineral medicine described in the oriental medicine book and mineral medicines applicable to atopic dermatitis treatment. Econ Environ Geol 2015;48(3):231-40.
- Yoo TM, KiM SS, RoH YN, Yi SY, Kim OH, Rheu HM, et al. General pharmacology of bamboo salt. J Appl Pharmacol 2000;8:93-8.
- He FJ, MacGregor GA. Salt: Flawed research should not divert actions to reduce intake. Nat Rev Nephrol 2016;12(9):514-5.
[Crossref] [Google Scholar] [PubMed]
- Ha JO, Park KY. Comparison of mineral contents and external structure of various salts. J Kor Soc Food Sci Nutr 1998;27:413-8.
- Kim HL, Lee SJ, Lee JH, Kim IC. Sulfur dioxide, mineral contents and physicochemical properties generated during manufacture of bamboo salt. J Kor Soc Food Sci Nutr 2014;43(8):1248-56.
- Yoou MS, Nam SY, Yoon KW, Jeong HJ, Kim HM. Bamboo salt suppresses skin inflammation in mice with 2, 4-dinitrofluorobenzene-induced atopic dermatitis. Chin J Nat Med 2018;16(2):97-104.
[Crossref] [Google Scholar] [PubMed]
- Kim DH, Lee KJ, Qi XF, Lee YM, Yoon YS, Kim JL, et al. The effects of magnesium rich sea mineral water on atopic dermatitis-like skin lesions in hairless mice. Appl Microscopy 2008;38(3):167-74.
- Yoon BI, Bae WJ, Ha US, Hong SH, Lee JY, Yoon KH, et al. The anti-inflammatory and antimicrobial effects of selenium-rich hot spring water on a chronic bacterial prostatitis rat model. Kor J Androl 2012;30(1):64-70.
- Choi EA, Kim JK, Kim KS, Choi JE, Cho CK, Lee YW, et al. Anticancer effects of egg white combined-chalcanthite on NCI-H460 tumor regression model. J Physiol Pathol Kor Med 2010;24(2):272-7.
- Lee TH, Song HK, Jang JY, Kim DY, Park HK, Choi EA, et al. Anti-inflammatory effect of egg white-chalcanthite and purple bamboo salts mixture on arthritis induced by monosodium iodoacetate in Sprague-Dawley rats. Lab Anim Res 2016;32(2):91-8.
[Crossref] [Google Scholar] [PubMed]
- Choi EA, Park HY, Yoo HS, Choi YH. Anti-inflammatory effects of egg white combined with chalcanthite in lipopolysaccharide-stimulated BV2 microglia through the inhibition of NF-κB, MAPK and PI3K/Akt signaling pathways. Int J Mol Med 2013;31(1):154-62.
[Crossref] [Google Scholar] [PubMed]
- Choi EA, Kim KH, Yoo BC, Yoo HS. Induction of apoptotic cell death by egg white combined-chalcanthite on NCI-H460 human lung cancer cells. J Pharmacopuncture 2009;12(3):49-59.