- *Corresponding Author:
- Sayani Bhattacharyya
Department of Pharmaceutics, Krupanidhi College of Pharmacy, Chikka Bellandur, Varthur, Bangalore 560035, India
E-mail: sayanibh@gmail.com
| Date of Received | 22 October 2021 |
| Date of Revision | 12 January 2022 |
| Date of Acceptance | 11 May 2022 |
| Indian J Pharm Sci 2022;84(3):575-585 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Etravirine, an antiretroviral agent, used in the treatment of human immunodeficiency virus belongs to biopharmaceutical classification system classification IV. The reported solubility of the drug is 0.0169 mg/ ml. In the present study, an attempt was made to enhance the solubility of etravirine by crystal engineering technique. The cocrystallization method was carried out using 12 different coformers and each coformer was studied in two different stoichiometric ratios. A preliminary screening of all the cocrystals was done by determination of melting point and solubility. A statistical evaluation of all the cocrystals on solubility was carried out at a significance level of p<0.05. The best cocrystals were subjected to drug content, in vitro drug release, solid-state study (fourier transform infrared spectroscopy, differential scanning calorimetry, powder x-ray diffraction) and stability study for 3 mo. Coformer benzoic acid showed a significant improvement in etravirine solubility in the drug:coformer ratio of 1:1 and 1:2. The drug:benzoic acid ratio of 1:2 was found to have more solubility and showed enhanced dissolution compared to pure drug. The in vitro dissolution rate of the drug:benzoic acid ratio of 1:2 was found to be more than 90 % in 60 min. Therefore, it can be concluded that the cocrystallization method with benzoic acid as coformer can be a promising approach for solubility improvement of etravirine.
Keywords
Etravirine, cocrystal, crystal engineering, solubility enhancement, dissolution
The crucial factors that determine the oral bioavailability of drugs are solubility and permeability. Among the biopharmaceutical factors, solubility of the drug is the key factor for the successful delivery of drugs, as it determines the systemic exposure in terms of dissolution particularly when administered orally. At the time of development and formulation, the pharmaceutical industries face major challenges in improving the bioavailability of the drugs having poor solubility. There are various methods available to improve the bioavailability of the drugs including micronization, nanonization, salt formation, micellar solubilizations and complexation, etc[1]. The modification of crystal habit of the Active Pharmaceutical Ingredient (API) either in amorphous form, anhydrous form or polymorphism suffers from many drawbacks in preserving the stability of the component, balancing hygroscopicity and removal of toxic solvents used in the crystallization process. Therefore, to fine-tune the API properties like solubility, stability and micromeritics, the co-crystallization process is found to be beneficial for the pharmaceutical industry[2]. Cocrystals are homogeneous crystalline materials comprised of at least two different components incorporated in the same crystal lattice with a defined stoichiometry[3]. Cocrystal structures are made up of noncovalent interactions between the API and the coformers which lead to the formation of supramolecular synthons[4]. The supramolecular assembly not only fine-tunes the physical properties like solubility, hygroscopicity and stability of the API in its crystal structure but also helps to address the flow property, compressibility and manufacturability.
Etravirine is an antiretroviral agent used in the treatment of Human Immunodeficiency Virus type 1 (HIV-1) infection. It is classified as a Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI). It belongs to Biopharmaceutical Classification System (BCS) class IV molecule. The problems associated with class IV include low aqueous solubility, poor permeability, significant food effect which lead to low and variable bioavailability. The reported solubility of etravirine is 0.0169 mg/ml[5]. Reported literature showed that the improvement of solubility of etravirine was studied by solid dispersion and spray drying methods[6,7]. The present study focuses on the development of cocrystals of etravirine using different coformers in different molar ratios and evaluating their effects on melting point, solubility, and percentage (%) drug release.
Materials and Methods
Etravirine was obtained as a gift sample from Apotex Pharmachem India Pvt. Ltd. Bangalore Karnataka. All other conformers and chemicals were of analytical grade, obtained from SD fine chemicals, Bangalore.
Method of preparation of etravirine cocrystal:
Etravirine cocrystals were prepared using twelve different coformers. The twelve different coformers used are mannitol, sodium saccharin, salicylic acid, tartaric acid, benzoic acid, urea, succinic acid, citric acid, ascorbic acid, caffeine, oxalic acid and magnesium stearate. The drug was mixed stoichiometrically with the coformers in a ratio of 1:1 and 1:2 in a mortar and pestle for 30 min. Few drops of 20 % w/v ethanol-water mixture were added during grinding[8]. The mixture was dried overnight at ambient temperature and stored in a desiccator[9]. The mixture was passed through sieve number 60 and stored in a glass vial for further studies.
Determination of melting point:
The melting point of the pure drug and its co crystals was determined by the capillary method. In this method, the powder sample was introduced into the capillary tube by gently tapping. The capillary tube was tied with a thermometer and was placed in the heated thiele tube containing liquid paraffin. The temperature was raised slowly until the transition happened to the liquid state. The temperature at which the sample started melting was recorded.
Determination of drug content:
The sample equivalent to 5 mg was dissolved in 25 ml of methanol. A quantity of 5 mg of drug was dissolved in 25 ml of methanol in a separate volumetric flask. The solutions were diluted suitably with methanol and the absorbance was estimated spectrophotometrically using methanol as blank at 311 nm. The % drug content was determined using the following formula. All findings were carried out in triplicates.
% Drug content=Absorbance×100/Standard absorbance
Determination of solubility:
The solubility of etravirine was determined by dissolving 25 mg equivalent of etravirine cocrystal in a flask containing 50 ml of distilled water. The solutions were vortexed for 2 min and placed afterward to a rotary shaker at 100 rpm for 48 h at room temperature. After that 0.5 ml slurry was withdrawn from each sample and filtered through 0.22 μm AST works disposable syringe filter. After suitable dilution with methanol, the concentration was measured spectrophotometrically at 311 nm. The solubility study was performed in triplicates for each sample to minimize the error[10].
Selection of dissolution media by estimation of Gibbs free energy:
The best cocrystals of etravirine were taken for solubility study in different dissolution media like 0.01 M hydrochloric acid solution, 1 % w/v Sodium Lauryl Sulfate (SLS) solution and phosphate buffer pH 7.4. Cocrystal equivalent to 100 mg was dispersed in 250 ml of the selected medium and placed in a rotary shaker for 48 h at room temperature. The solubility of the samples in different media was determined after suitable dilution. From the solubility data of the cocrystals in different media, Gibbs free energy was calculated using the following equation where RT=Ideal gas constant, S0=Solubility of coformers in the selected media and SS=Solubility of etravirine in the selected media[11].
ΔG=-2.303 RT log S0/SS
Drug release study:
The dissolution study was carried out for the best cocrystals of etravirine in 900 ml of 1 % SLS in United States Pharmacopeia (USP) paddle-type II apparatus. An equivalent of 100 mg etravirine was used to study the release from the cocrystals. The stirring speed was maintained at 50 rpm and the bath temperature was kept at 37°±1°. The dissolution was carried out for 60 min. The sample was withdrawn from time to time and replaced with a fresh solution of media. The samples after dilution with methanol were analyzed spectrophotometrically at 311 nm. The analysis for each study point was performed in triplicates[7].
Particle size determination:
The particle size determination of the best cocrystals was carried out in Malvern zeta sizer at a detection angle of 173° at 25°. A specific amount of sample was dispersed in millipore water (2 mg/ml) and sonicated for 10 min. The sample was further diluted with water and scanned to determine the particle size.
Fourier Transform Infrared (FTIR) spectroscopy study:
The cocrystals of etravirine were taken for interaction study. The sample was analyzed using FTIR 8400S device Shimadzu, Japan using the Potassium Bromide (KBr) pellet technique from a range of 4000 cm-1 to 400 cm-1. The spectra were generated for the pure drug with the best coformer in both the ratio of 1:1 and 1:2[12].
Differential Scanning Calorimetry (DSC) study:
The thermal analysis of the samples was carried out using DSC 60 (Shimadzu) for the pure drug and the best cocrystals in both ratios. Around 5 mg sample was placed in the aluminum pan under a nitrogen flow rate of 20 ml/min, with a gradual increase in temperature by 10o per min from 30° to 300°. An empty aluminum pan was used as a reference standard. The thermograms and the phase transition behavior were recorded for further analysis[13].
Powder X-Ray Diffraction (PXRD):
PXRD diffraction pattern of the pure drug and the different ratios of the best cocrystal were studied in the Bruker D8 diffractometer instrument. A Copper K-alpha 1 (Cu K-α 1) tube was used as the source and the instrument was set at 40 kV and 30 mA. A scan was carried out from 5º to 59.98º 2θ at 2θ scan step of 0.03º at a scan step time of 0.8 s. The diffraction patterns were recorded for further analysis of the reflection angle and peak intensity[8].
Stability studies:
The best cocrystals were stored in sealed glass vials and kept at 40°±2° at a relative humidity of 75 %±5 % for 3 mo. The samples were analyzed for solubility, drug content and in vitro drug release every 30 d[9].
Results and Discussion
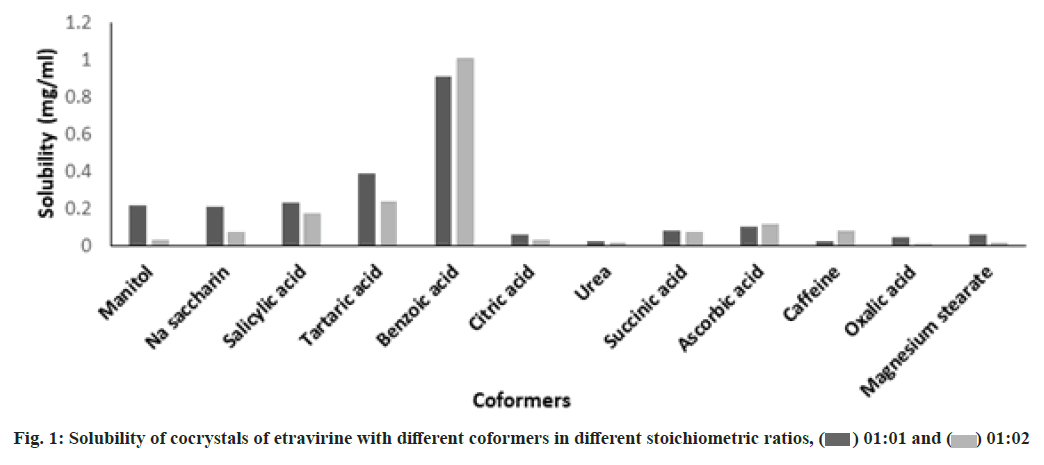
Crystal engineering of poorly soluble drug etravirine was carried out by screening of different coformers. The solvent drop grinding method was used to prepare different cocrystals of etravirine. The melting point of each formulation was determined to estimate the entropy of the crystal lattice of the cocrystals. The melting point of the cocrystals of etravirine with various coformers in 1:1 and 1:2 ratios is listed in Table 1. The pure drug showed a melting point of 265°. It was found that all the coformers in the stoichiometric ratio could bring down the melting point of the cocrystals. Among them, the coformer benzoic acid showed the least melting point of the cocrystal of etravirine in both the stoichiometric ratios. This lowering of melting point is an indication of the asymmetric molecular structure of the cocrystals. The drug content for all the cocrystals was found to be within the range of 88 % to 91 % as shown in Table 1.
| Coformer | Code for ratio 1:1 | Melting point of cocrystal (1:1) (°) | Solubility of cocrystal (1:1) (mg/ml) | Drug content (%) | Code for ratio 1:2 | Melting point of cocrystal (1:2) (°) | Solubility of cocrystal (1:2) (mg/ml) | Drug content (%) |
|---|---|---|---|---|---|---|---|---|
| D-Mannitol | ETM1 | 249±0.05 | 0.221±0.004 | 90.01±4.50 | ETM2 | 251±0.02 | 0.031±0.001 | 88.23±1.76 |
| Sodium saccharin | ETSS1 | 257±0.03 | 0.23±0.015 | 89.21±1.09 | ETSS2 | 283±0.06 | 0.076±0.001 | 89.27±2.08 |
| Salicylic acid | ETSA1 | 212±0.05 | 0.231±0.016 | 89.53±2.13 | ETSA2 | 187±0.03 | 0.181±0.011 | 88.52±3.60 |
| Tartaric acid | ETTA1 | 209±0.02 | 0.398±0.011 | 90.45±5.01 | ETTA2 | 191±0.03 | 0.255±0.020 | 90.97±2.34 |
| Benzoic acid | ETBA1 | 169±0.03 | 0.920±0.017 | 90.61±3.52 | ETBA2 | 176±0.06 | 1.011±0.013 | 89.93±2.06 |
| Citric acid | ETCA1 | 177±0.07 | 0.066±0.005 | 89.45±2.66 | ETCA2 | 187±0.06 | 0.034±0.001 | 88.66±4.27 |
| Urea | ETU1 | 183±0.04 | 0.022±0.001 | 89.88±2.90 | ETU2 | 167±0.08 | 0.018±0.001 | 89.10±2.54 |
| Succinic acid | ETSU1 | 176±0.05 | 0.078±0.001 | 91.03±5.12 | ETSU2 | 184±0.06 | 0.075±0.004 | 88.54±2.98 |
| Ascorbic acid | ETAA1 | 197±0.02 | 0.117±0.016 | 90.78±1.45 | ETAA2 | 196±0.02 | 0.123±0.007 | 88.44±1.38 |
| Caffeine | ETC1 | 182±0.06 | 0.029±0.015 | 90.09±3.06 | ETC2 | 213±0.04 | 0.083±0.002 | 90.67±2.71 |
| Oxalic acid | ETOA1 | 196±0.06 | 0.046±0.001 | 89.11±3.22 | ETOA2 | 190±0.06 | 0.084±0.002 | 90.40±2.13 |
| Magnesium stearate | ETMS1 | 249±0.04 | 0.070±0.008 | 89.31±4.12 | ETMS2 | 269±0.06 | 0.019±0.001 | 89.54±2.04 |
Note: *All the values are mean±Standard Deviation (SD) (n=3)
Table 1: Melting Point and Solubility of Etravirine With Different Coformers
The solubility study of the cocrystals revealed that the solubility of etravirine was increased with all the coformers compared to the pure drug. The solubility data of the different cocrystals were compared using Dunnett’s test at a significant level p<0.05 using GraphPad Prism 5 software. It was found that the effect of different coformers on etravirine solubility was statistically significant in 1:1 ratio of drug and coformer except for urea and caffeine as mentioned in Table 2. The solubility was not found statistically significant for mannitol, citric acid and urea at a drug and coformer ratio 1:2. These differences in observation might be attributed to the mismatch of structural fit between the drug and coformers[14]. The solubility study revealed that among the 12 different coformers, benzoic acid was found to increase the solubility of the drug in water significantly as represented in fig. 1. The drug and benzoic acid at a stoichiometric ratio of 1:1 and 1:2 showed a drastic increase in the solubility of the drug. An approximately 54-folds and 60-folds increase in aqueous solubility was observed in cocrystals (ETBA1 and ETBA2) respectively. Therefore, the cocrystals ETBA1 and ETBA2 were taken for further evaluation.
| Dunnett’s multiple comparison test | Drug:Coformer=1:1 | Drug:Coformer=1:2 | ||||
|---|---|---|---|---|---|---|
| Mean difference | q | Significant, p<0.05 | Mean difference | q | Significant, p<0.05 | |
| Pure drug vs. mannitol | -0.2044 | 25.1 | Yes | -0.01528 | 2.384 | No |
| Pure drug vs. sodium saccharin | -0.2119 | 26.03 | Yes | -0.05899 | 9.207 | Yes |
| Pure drug vs. salicylic acid | -0.2163 | 26.56 | Yes | -0.1651 | 25.76 | Yes |
| Pure drug vs. tartaric acid | -0.382 | 46.91 | Yes | -0.2384 | 37.2 | Yes |
| Pure drug vs. benzoic acid | -0.9031 | 110.9 | Yes | -0.9873 | 154.1 | Yes |
| Pure drug vs. citric acid | -0.04996 | 6.136 | Yes | -0.01689 | 2.635 | No |
| Pure drug vs. urea | -0.005013 | 0.6157 | No | -0.00157 | 0.2455 | No |
| Pure drug vs. succinic acid | -0.0621 | 7.627 | Yes | -0.05855 | 9.138 | Yes |
| Pure drug vs. ascorbic acid | -0.1001 | 12.3 | Yes | -0.1061 | 16.56 | Yes |
| Pure drug vs. caffeine | -0.01223 | 1.502 | No | -0.0666 | 10.39 | Yes |
| Pure drug vs. oxalic acid | -0.02904 | 3.566 | Yes | -0.06673 | 10.41 | Yes |
Note: *q is the difference between the two means (D) divided by the standard error of that difference
Table 2: Dunnett’s Test
To establish the solubility in different dissolution media, a 100 mg dose equivalent of ETBA1 and ETBA2 were tested for solubility in 0.01 M hydrochloric acid, 1 % w/v SLS solution and phosphate buffer 7.4. The study revealed that the solubility of both the cocrystals was high in 1 % w/v SLS solution and resulted in minimum Gibbs free energy, presented in Table 3. Therefore the dissolution study of the cocrystals was carried out in 1 % w/v SLS solution.
| Medium | Solubility (mg/ml) | ETBA1 DG (kJ/mol) |
Solubility (mg/ml) | ETBA2 DG (kJ/mol) |
|---|---|---|---|---|
| 0.01 M hydrochloric acid | 0.396±0.12 | -7.81±0.01 | 0.413±0.14 | -7.92±0.04 |
| 1 % w/v SLS solution | 1.98±0.25 | -11.80±0.05 | 2.478±0.18 | -12.35±0.03 |
| Phosphate buffer pH 7.4 | 0.594±0.11 | -8.82±0.02 | 0.619±0.43 | -8.92±0.04 |
Note: *All the values are mean±SD (n=3)
Table 3: Selection Of Dissolution Medium
The release study of etravirine from the selected cocrystals of benzoic acid was compared with the equivalent amount of pure drug of etravirine in the same dissolution condition. The % cumulative drug release vs. time graph is presented in fig. 2. The pure drug showed a release of 10 % in 10 min and 41 % over 60 min. But both the cocrystals ETBA1 and ETBA2 showed an improved drug release in 10 min, and finally and approximately 86 % and 93 % of drug release for 60 min was observed for ETBA1 and ETBA2 respectively. The cocrystal ETBA2 showed higher release compared to ETBA1. The high release may be due to the formation of a weaker crystalline structure with the stoichiometric proportion of the coformer. The greater dissolution of the cocrystals over pure etravirine proved the enhancement of solubility of etravirine in presence of coformer.
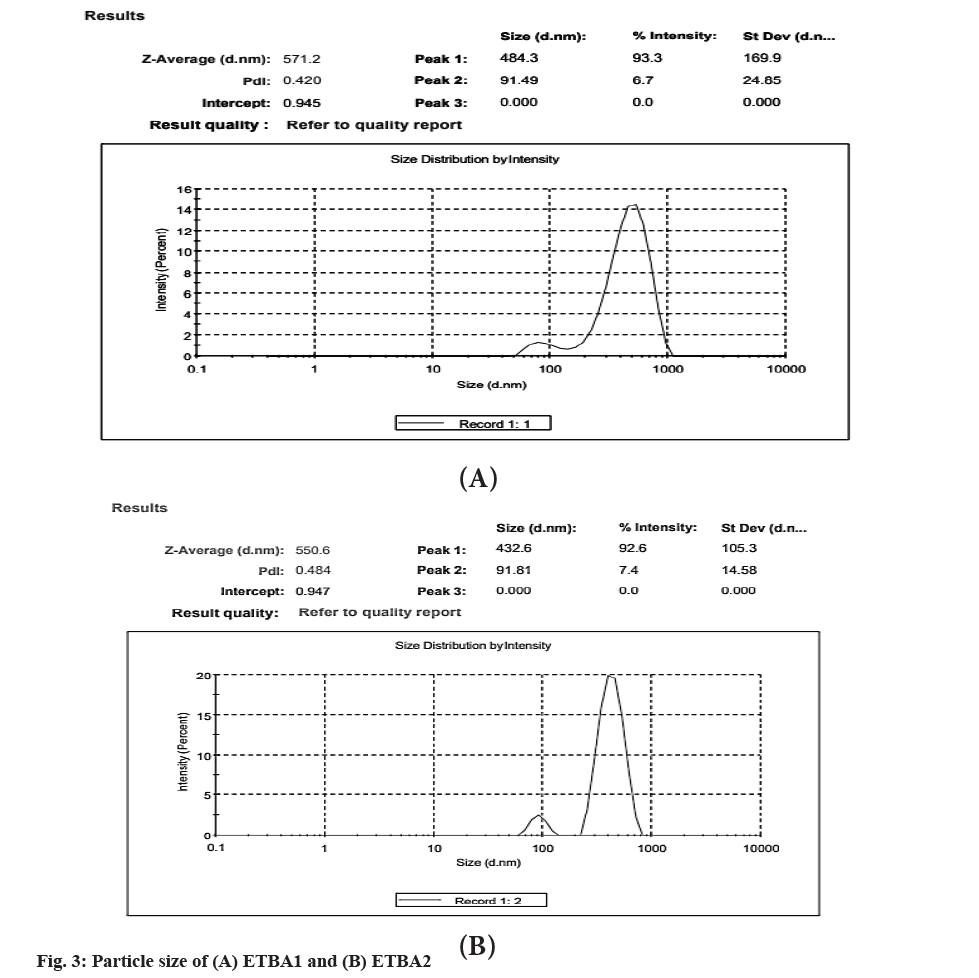
The particle size of the formulation ETBA1 and ETBA2 was found to be 571.2 and 550.6 nm respectively as shown in fig. 3. The nano-size range of the cocrystal also confirms the high rate of dissolution.
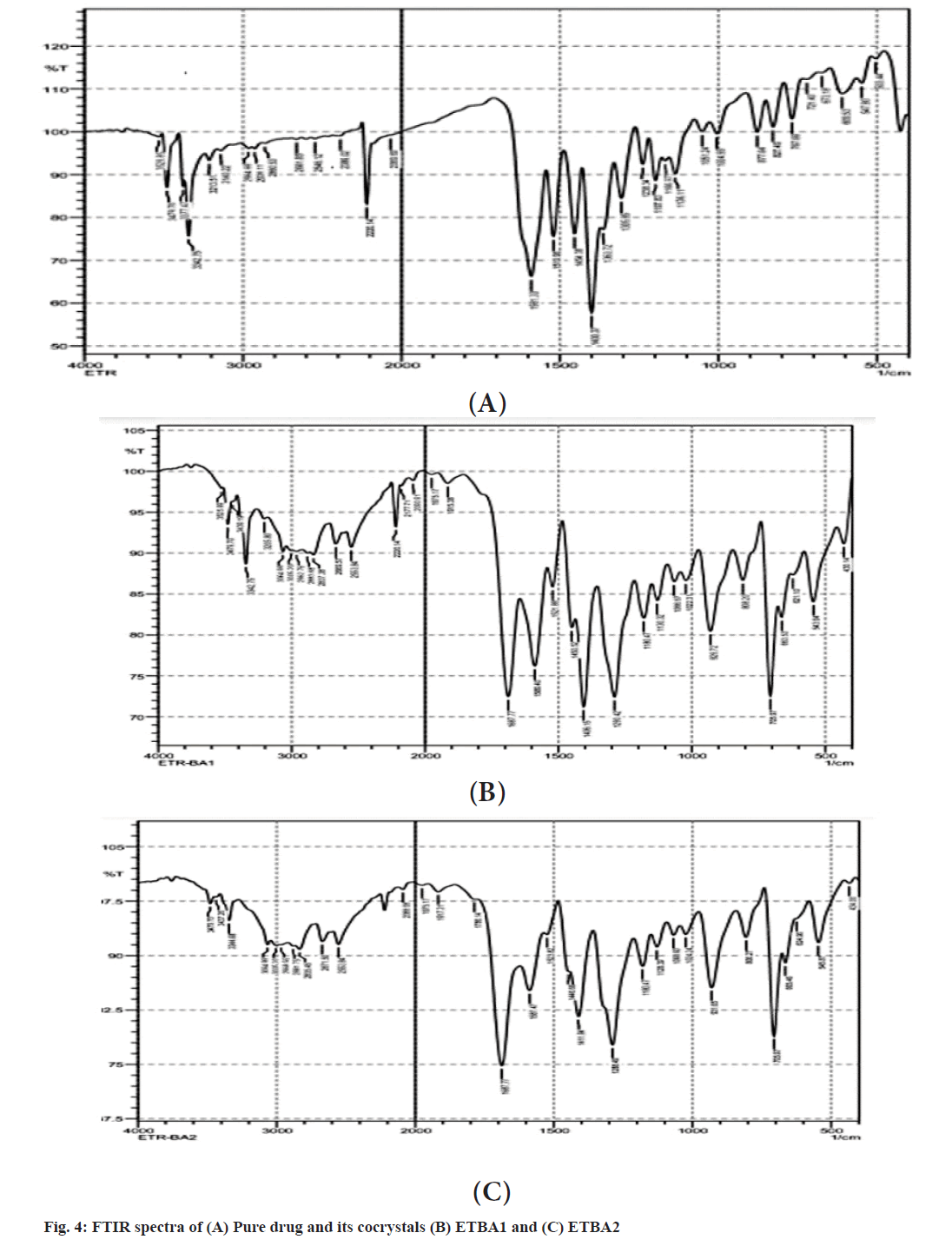
The FTIR spectra of pure drug and cocrystals of benzoic acid in different ratios are shown in fig. 4. The pure drug showed the characteristic peaks at 2220 cm-1 for the presence of aromatic -C≡N group, at 1305 cm-1 for the presence of C-O stretching, at 1238 cm-1 for the C-N stretching and a C-Br stretching at 684 cm-1[15]. All the characteristic peaks are well preserved in the formulations ETBA1 and ETBA2. The spectra revealed neither appearance nor disappearance of any characteristic peaks of etravirine in the cocrystals. Therefore, it indicates that there is no incompatibility or interaction between the drug and benzoic acid. The stretching of -C≡N group in ETBA1 and ETBA2 indicated the formation of H bonding between etravirine and benzoic acid and the formation of an amorphous form[16].
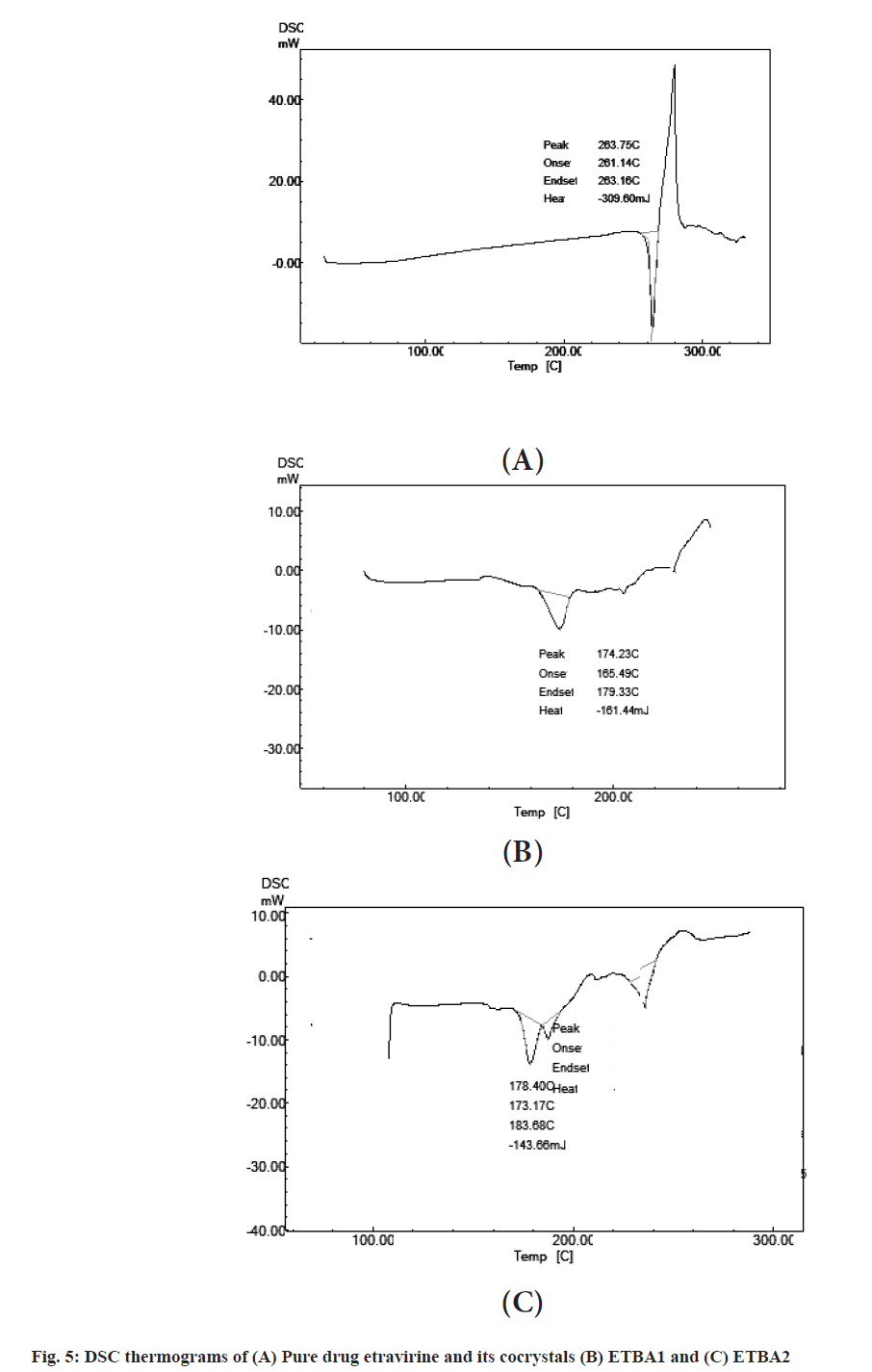
The DSC thermograms are presented in fig. 5. Thermograms revealed that the phase transition of the pure drug occurred in the range of 263.75°. The DSC thermograms showed a shift of peak to 174° and 178° for the formulations ETBA1 and ETBA2 respectively. This shift might be due to the strong non-covalent interaction between etravirine and benzoic acid. Therefore, a new crystalline arrangement might have formed which had altered the physiochemical property of the drug inside the cocrystal that resulted in reduced melting point and improved solubility.
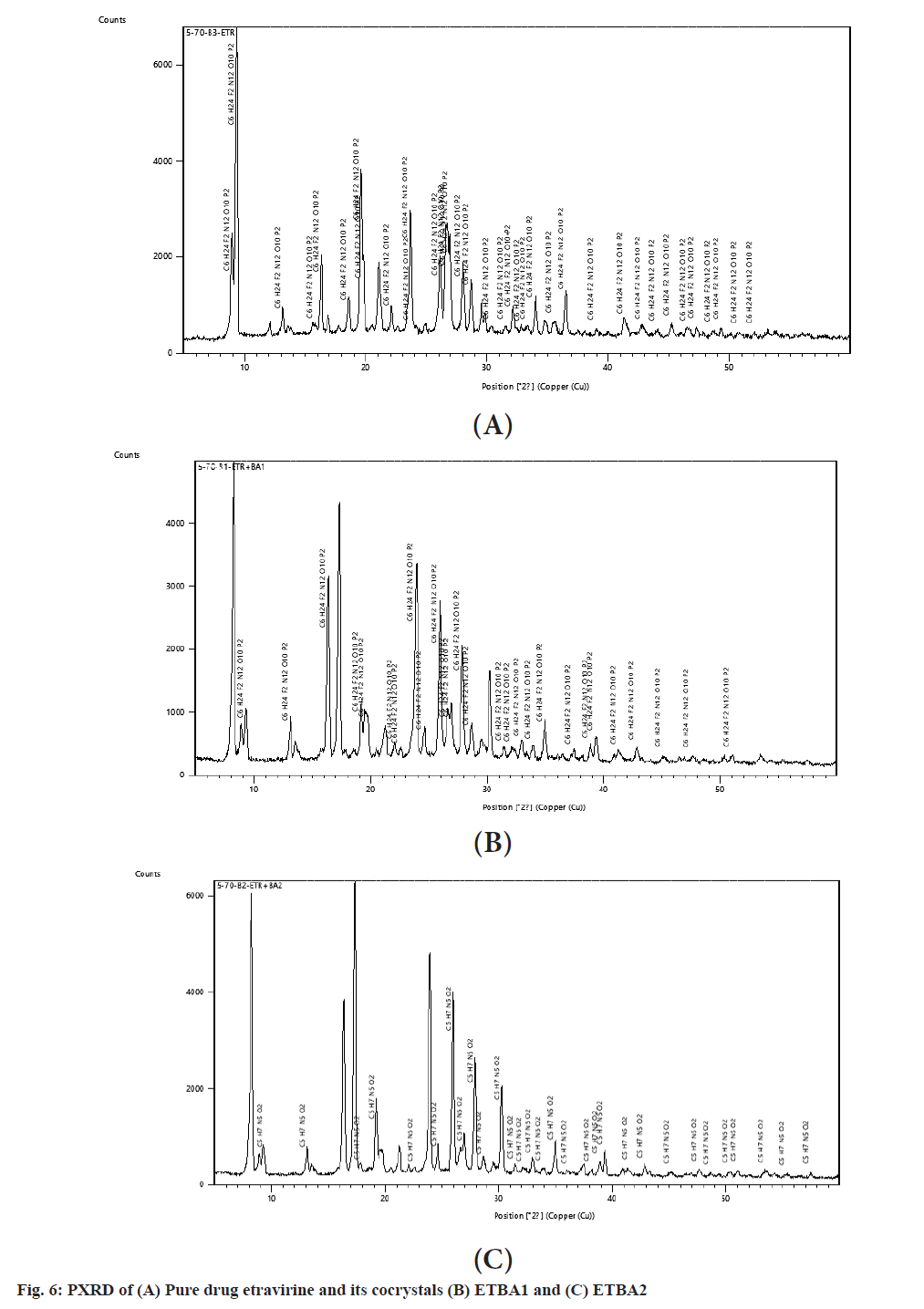
The peak intensities of pure drug and the cocrystals of benzoic acid at various diffraction angles are represented in Table 4 and fig. 6. The diffraction pattern of the pure drug exhibited peaks of high intensities and indicated the crystalline form of the drug which was in confirmation with the DSC results. The PXRD pattern of the cocrystals also revealed that all the major peaks of the pure drug were preserved and confirmed the observations of compatibility study with FTIR[15]. The PXRD pattern of the physical mixture of the cocrystals showed the appearance of broader peaks with low intensities compared to the pure drug. The low intensities of the peaks were attributable to the effect of coformer in altering the physicochemical properties of etravirine. The lowest intensities of the peaks in the formulation ETBA2 indicated a strong transformation of the crystallinity of the pure drug and that might have led to the increase in solubility and improved dissolution.
| Etravirine (2θ) | % Intensities | ETBA1 (2θ) | % Intensities | ETBA2 (2θ) | % Intensities |
|---|---|---|---|---|---|
| 9.35 | 100 | 9.3 | 16.46 | 9.32 | 7.99 |
| 19.59 | 51.6 | 19.65 | 16.65 | 19.58 | 10.05 |
| 22.1 | 9.14 | 22.07 | 5.3 | 22.07 | 2.52 |
| 23.7 | 42.68 | 23.89 | 3.03 | 23.57 | 1.43 |
| 26.06 | 24.29 | 26.61 | 16.66 | 26.62 | 7.06 |
| 28.66 | 19.28 | 28.62 | 12.2 | 28.65 | 4.64 |
| 29.53 | 12.04 | 29.54 | 6.06 | 29.59 | 2.55 |
| 32.11 | 8.59 | 32.96 | 6.47 | 32.17 | 1.82 |
| 36.49 | 17.13 | 37.4 | 3.09 | 36.1 | 0.83 |
| 45.19 | 4.71 | 45.09 | 2.07 | 45.14 | 1.15 |
| 47.27 | 3.51 | 47.58 | 1.92 | 48.63 | 1.09 |
| 9.35 | 100 | 9.3 | 16.46 | 9.32 | 7.99 |
| 19.59 | 51.6 | 19.65 | 16.65 | 19.58 | 10.05 |
Table 4: Peak Intensities of Pure Drug and The Cocrystals of Benzoic Acid
The above characterizations of the cocrystals of etravirine and its improved solubility can be further supported by the acid dissociation constant (ΔpKa) of the drug and the coformer. The Food and Drug Administration (FDA) guidance for the industry also says that the difference in pKa between drug and coformer should be <1, to be classified as cocrystal[17]. In the present study, the ΔpKa of the drug and coformer benzoic acid was found to be less than 1.
The 3 mo stability study of the cocrystals indicated that there were no major changes in the solubility, drug content and release of drug as indicated in Table 5. Both the cocrystals were found to be stable at the storage condition of 40°±2° at a relative humidity of 75 %.
| Days | ETBA1 | ETBA2 | ||||
|---|---|---|---|---|---|---|
| Solubility (mg/ml) | % Drug content | % Drug release | Solubility (mg/ml) | % Drug content | % Drug release | |
| 30 | 0.91±0.012 | 88.0±2.13 | 85.87±1.10 | 0.989±0.015 | 90.02±1.05 | 92.89±2.11 |
| 60 | 0.90±0.02l | 87.67±1.16 | 85.6±1.15 | 0.975±0.01 | 89.95±0.90 | 92.65±1.25 |
| 90 | 0.89±0.011 | 87.60±1.01 | 85.34±1.21 | 0.97±0.016 | 89.9±1.12 | 92.43±1.16 |
Note: *All the values are mean±SD (n=3)
Table 5: Stability Study of ETBA1 and ETBA2
In conclusion, the crystal engineering of etravirine with coformers can be regarded as an effective method in improving solubility and dissolution of etravirine. In the present research, a screening of twelve different coformers was carried out. Among them, benzoic acid was found to be the most promising coformer based on the solubility study. The amount of benzoic acid in the cocrystals was also kept within the Generally Recognized As Safe (GRAS) limit[18]. The observation from the compatibility, thermal and surface characteristics study supported the formation of cocrystal of etravirine with benzoic acid. The improvement in solubility and dissolution of etravirine was attributed to the altered solid-state characteristics of the drug in the coformer. The significant improvement in the water solubility resulted in high drug release in the dissolution medium of 1 % w/v SLS solution. The results from the stability study indicated the firmness of the products. Therefore, it can be concluded that the co-crystallization approach and use of benzoic acid as a coformer can be suitably employed to improve the solubility of etravirine.
Acknowledgements:
Authors are highly obliged to the management and principal of Krupanidhi College of Pharmacy, Bangalore for providing the necessary infrastructure to conduct the research work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. Int Sch Res Notices 2013;2013:1-16.
[Crossref] [Google Scholar] [PubMed]
- Kumar S. Pharmaceutical cocrystals: An overview. Indian J Pharm Sci 2018;79(6):858-71.
- Patole T, Deshpande A. Co-crystallization-A technique for solubility enhancement. Int J Pharm Sci Res 2014;5(9):3566-76.
- Gadade DD, Pekamwar SS. Pharmaceutical cocrystals: Regulatory and strategic aspects, design and development. Adv Pharm Bull 2016;6(4):479-94.
[Crossref] [Google Scholar] [PubMed]
- DrugBank accession number DB06414. Etravirine. DrugBank online; 2022.
- Ramesh K, Shekar BC, Khadgapathi PO. Formulation and evaluation of poorly soluble etravirine by spray drying method. Int J Pharm Pharm Sci 2015;7:98-103.
- Ramesh K, Shekar BC, Khadgapathi P, Bhikshapathi DV, Gourav N. Enhancement of solubility and bioavailability of etravirine solid dispersions by solvent evaporation technique with novel carriers. IOSR J Pharm Biol Sci 2015;10(4):30-41.
- Al-Kazemi R, Al-Basarah Y, Nada A. Dissolution enhancement of atorvastatin calcium by cocrystallization. Adv Pharm Bull 2019;9(4):559-70.
[Crossref] [Google Scholar] [PubMed]
- Panzade P, Shendarkar G. Superior solubility and dissolution of zaltoprofen via pharmaceutical cocrystals. Turk J Pharm Sci 2019;16(3):310-6.
[Crossref] [Google Scholar] [PubMed]
- Alavi M, Karimi N, Safaei M. Application of various types of liposomes in drug delivery systems. Adv Pharm Bull 2017;7(1):3-9.
- Bhatia M, Devi S. Development, characterisation and evaluation of PVP K-30/PEG solid dispersion containing ketoprofen. Acta Pharm Sci 2020;58(1):83-99.
- Shashidhar GM, Manohar B. Nanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique. RSC Adv 2018;8(60):34634-49.
- Shinde U, Desai H, Martis EA, Amin P. Co-crystals of gliclazide: Formulation and characterisation. Am J PharmTech Res 2017;7(2):217-35.
- Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 2007;59(7):617-30.
[Crossref] [Google Scholar] [PubMed]
- Kommavarapu P, Maruthapillai A, Palanisamy K, Teja Koya R. Physical characterization and dissolution performance assessment of etravirine solid dispersions prepared by spray drying process. Pak J Pharm Sci 2016;29(6):2023-31.
[Google Scholar] [PubMed]
- Allu S, Suresh K, Bolla G, Mannava MC, Nangia A. Role of hydrogen bonding in cocrystals and coamorphous solids: Indapamide as a case study. CrystEngComm 2019;21(13):2043-8.
- Rodrigues M, Baptista B, Lopes JA, Sarraguça MC. Pharmaceutical cocrystallization techniques. Advances and challenges. Int J Pharm 2018;547(1):404-20.
[Crossref] [Google Scholar] [PubMed]
- U.S. Environmental protection agency, National center for environmental assessment. Benzoic acid ; CASRN 65-85-0. Integrated Risk Information System (IRIS), Chemical Assessment Summary; 1988:1-10.
 ) 01:01 and (
) 01:01 and (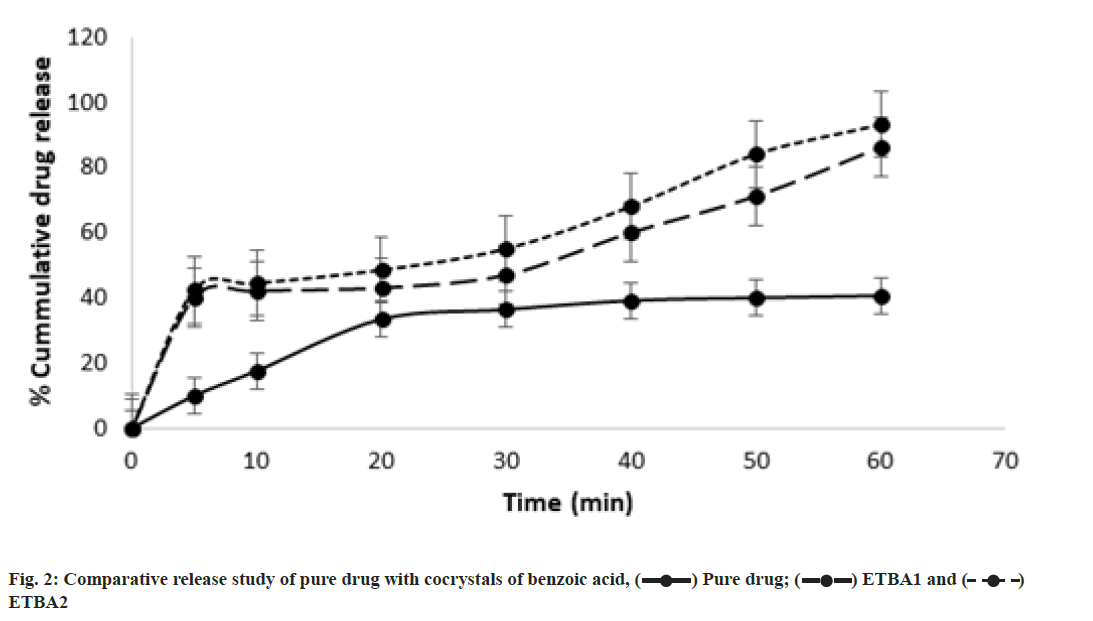
 ) Pure drug; (
) Pure drug; ( ) ETBA1 and (
) ETBA1 and ( )
ETBA2
)
ETBA2