- *Corresponding Author:
- Mahmoud Mansour
Department of Basic Pharmaceutical Sciences, College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Ministry of the National Guard-Health Affairs, Riyadh 11481, Saudi Arabia
E-mail: mansoura@ksau-hs.edu.sa
| This article was originally published in a special issue,“Integrative Approaches in Biomedical Sciences for Drug Discovery and Development” |
| Indian J Pharm Sci 2024:86(6) Spl Issue “92-98” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Several techniques, such as high-performance liquid chromatography and ultraviolet-visible spectroscopy, have been developed and validated for analyzing allopurinol in various pharmaceutical formulations. In this study, the validated method was applied to analyze two allopurinol products available in the market (No-Uric, and Loric). The chromatographic separation involved a mobile phase consisting of a 50:50 ratio of acetonitrile to buffer 4.6 v/v. The separation process utilized a C18 rapid resolution column (4.6×100 mm, 3.5 μm, agilent high-performance liquid chromatography column). The λmax of allopurinol was found to be at 254 nm. Separation was achieved using a 20 μl injection volume with a run time of 3.0 min. The method exhibited linearity within a concentration range of 2.5-15 μg/ ml for allopurinol. Method validation was performed in accordance with International Council for Harmonization guidelines, including evaluations of specificity, selectivity, linearity, accuracy, precision, lower limit of quantification and lower limit of detection. The established lower limit of quantification and lower limit of detection for allopurinol were 4.09 μg /ml and 1.36 μg /ml, respectively. The percentage assay for all pharmaceutical products containing allopurinol met the acceptance criteria range of 90 %-110 %. These methodologies proved effective for the accurate quantitative analysis of allopurinol in pharmaceutical formulations.
Keywords
Allopurinol, validation, percentage assay, high performance liquid chromatography
Allopurinol is an isomer of hypoxanthine, and its active metabolite, oxipurinol, inhibits the xanthine oxidase enzyme, which converts xanthine and hypoxanthine into uric acid. It is widely used as a primary treatment for hyperuricemia (gout) associated with cancers, leukemia, and diuretic use. Allopurinol can be administered either orally or intravenously. The bioavailability of oral administration ranges from 67 % to 90 %, with peak plasma concentrations typically occurring within 1 h. The volume of distribution is approximately 1.6 l/ kg. Allopurinol is metabolized by aldehyde oxidase into its active form, oxipurinol[1,2]. Peak plasma concentrations of oxipurinol are reached within 3 h to 5 h, while the mean elimination half-life is between 1 h to 1.5 h for allopurinol and 18 h to 40 h for oxipurinol. Approximately 10 % of allopurinol is excreted unchanged in the urine, 70 % is excreted as oxipurinol, and 20 % is eliminated in the feces. In patients with renal impairment (creatinine clearance <80 ml/min), the initial allopurinol dosage should be adjusted based on the estimated creatinine clearance. Additionally, the maintenance dose should be reduced to avoid toxic effects caused by elevated oxipurinol serum levels[3,4]. Therapeutic serum oxipurinol values are considered to range from 5 to 15 μg/ml[5] (fig. 1).
Co-administration of uricosuric drugs, such as probenecid and benzbromarone, can enhance the renal excretion of oxipurinol by interacting with the Urate Transporter 1 (URAT1) transporter[6,7]. In the treatment of patients with severe gout, a combination of these uricosuric drugs and allopurinol is commonly utilized[8,9]. Several methods have been described for the analysis of allopurinol and oxipurinol in human serum and pharmaceutical formulations. However, many of the published methods, particularly those using Reversed Phase High- Performance Liquid Chromatography (RP-HPLC), have notable limitations[10-18]. These include a lack of information regarding interference from concomitant medications commonly used by gout patients, upper limits of quantification in High Performance Liquid Chromatography (HPLC) chromatograms that do not cover the concentration range of allopurinol typically seen in clinical practice, and the absence of stability data for allopurinol and oxipurinol in serum and other formulations. Dastiagiriamma et al.[19] developed an HPLC method for the quantification of allopurinol and lesinurad in both the Active Pharmaceutical Ingredient (API) and marketed formulations. Many of the published methods, particularly those using RP-HPLC, have significant limitations. These include insufficient information on interference from concomitant medications commonly used by gout patients, inadequate upper limits of quantification in HPLC chromatograms that do not encompass the clinically relevant concentration range of allopurinol, and a lack of stability data for both allopurinol and oxipurinol in serum and other formulations[20]. Another several methods for allopurinol analysis have been developed, including spectrophotometric method[21], conventional volumetric titration method[22], and capillary electrophoresis[23,24].
However, the existing methods are not always practical, particularly for quantifying allopurinol in various pharmaceutical formulations. When compounds like oxipurinol are present, these methods may be unsuitable or time-consuming, often involving derivatization steps and extended chromatographic run times. Allopurinol has also been quantified in different pharmaceutical preparations using differential pulse polarography, and a flow injection technique has been developed for its analysis[25]. However, these methods often fail to achieve good recovery when applied to real samples[26]. Other methods are quite complicated and time-consuming, making them less ideal for practical purposes, especially for analyzing large numbers of samples. Only a few spectrophotometric assays have been reported for the detection of allopurinol in tablet formulations, typically based on the chemical reaction between allopurinol and another compound to form a colored complex.
Khayoon et al.[21] developed a simple and rapid spectrophotometric method for determining allopurinol in pharmaceutical tablet formulations. This method relies on the chemical interaction between allopurinol, catechol reagent, and Ferrous Fe(II), resulting in the formation of a blue soluble complex that is measured at a λmax of 580 nm. The method is effective for concentrations ranging from (2-10) μg/ml, with the color complex remaining stable for up to 2 h. This approach was successfully applied to allopurinol determination in pharmaceutical preparations. More recently, Bakr et al.[27] developed a new spectrophotometric method for quantifying allopurinol. This method involves an oxidative coupling reaction using 2-nitrophenol reagent and N-bromo succinimide, resulting in a yellow-colored product that exhibits the highest absorption at 420 nm, with a concentration range of (2.5-15) μg/ml. The aim of the present work was to develop a simple, cost-effective, rapid, and sensitive spectrophotometric method for determining allopurinol in pharmaceutical formulations (tablets) and to compare the sensitivity of this method with all previously reported methods. In addition, we will modify a new HPLC method for allopurinol using different column and mobile phase.
Materials and Methods
Materials:
Allopurinol standard powder (allopurinol, Glentham, United Kingdom) was used to prepare the standard stock solution and serial dilutions.
Reagents and chemicals:
Acetonitrile CHROMASOLVTM for HPLC (≥99.9 %) was procured from Honeywell (United States of America (USA), ultra-pure water was obtained from a Milli-Q® Integral 5 (ZRXQ005T0) system (Millipore®, USA), and sodium acetate was sourced from Loba Chemie, India.
Chromatographic conditions:
HPLC (1260 Infinity, Agilent Technologies) with a Variable Wavelength Detector (VWD) was used for method development and validation of allopurinol. Data acquisition, recording, and chromatographic integration were performed using the instrument software (OpenLab ChemStation).
Mobile phase preparation:
The aqueous mobile phase consists of a mixture of acetonitrile and buffer (4.6, 50:50, v/v) under isocratic conditions. The mobile phase was filtered through a 0.45 μm pore size filter, then sonicated and degassed in an ultrasonic bath (CPX5800H-E, Bransonic®).
Preparation of stock standard solution:
Preparing a standard allopurinol solution involved dissolving 1000 mg of allopurinol standard powder in water. Gently shaking the flask ensured the complete dissolution of the solution. The resulting concentration of the allopurinol stock solution was 1 mg/ml[18]. Preparing a standard allopurinol solution involved dissolving 1000 mg of allopurinol standard powder in water. Gently shaking the flask ensured the complete dissolution of the solution. The resulting concentration of the allopurinol stock solution was 1 mg/ml[18].
Preparation of standard serial dilution:
Preparation of standard calibration curve samples: Stock standard solutions of allopurinol (1 mg/ml) were diluted with mobile phase to get the calibration standard solutions over the range of (2.5-15) μg/ml to complete volume to 100 ml[19]. The glass vial (2 ml screw vial, 8 mm, Thermo Fisher Scientific Inc.) was filled with the standard solution and then injected into the HPLC system.
Preparation of quality control samples: A stock standard solution of allopurinol (1.00 mg/ml) were diluted with mobile phase to prepare low, medium, and high-quality control samples over the range of (2.5-15) μg/ml to complete volume to 100 ml[6]. Each serial solution was then mixed thoroughly, and the final concentrations were 6, 12.5, and 20 μg/ml (with two solutions prepared for each concentration). The glass vial (2 ml screw vial, 8 mm, Thermo Fisher Scientific Inc.) was filled with the standard solution and injected into the HPLC system.
Preparation of pharmaceutical samples assay test of tablet formulation: 10 tablets of No-Uric and Loric-100 were accurately weighed separately using an analytical balance, and the average weights were recorded. The tablets of each product were then crushed separately into a fine powder, and the weight equivalent to the average weight of a tablet was taken[6]. The powder was transferred to a volumetric flask and diluted up to the mark with the mobile phase. The concentration of the allopurinol assay test solution was 1 mg/ml for all pharmaceutical formulations. The sample was then immediately filtered through a syringe filter (0.45 μm). A 10 μl sample was pipetted using a micropipette 100 μl (Eppendorf Research® Plus) into a 100 ml amber volumetric flask and diluted to the mark with the mobile phase, resulting in a final concentration of 100 μg/ml. 3 solutions were prepared for analysis. 5 glass vials (2 ml screw vial, 8 mm, Thermo Fisher Scientific Inc.) were labeled, and 20 μl of the sample was injected into the HPLC system[6].
Method development, optimization, and validation: This paper describes the development and validation of an HPLC method for the determination of allopurinol in pharmaceutical formulations.
RP-HPLC method development:
A simple, sensitive, precise, and accurate RPHPLC method was developed for the estimation of allopurinol. Several mobile phases and columns were initially tested to have an appropriate chromatogram. The suitable column and the mobile phase used in the optimized method have been determined based on selectivity, sensitivity, and acceptable chromatographic parameters of the produced peaks in terms of peak sharpness, peak symmetry, and tailing factor. The chromatographic separation of allopurinol was achieved using a ZORBAX Eclipse Plus C18 rapid resolution column (4.6×100 mm, 3.5 μm, agilent). The detection wavelength was set to 254 nm, and the mobile phase consisted of a 50:50 (v/v) mixture of acetonitrile and pH 4.6 buffer, operated under isocratic conditions with a flow rate of 1 ml/min. A 20 μl injection volume was used for separation at 25°, with a total run time of 3 min.
Method validation:
The proposed method was validated according to the International Council for Harmonisation (ICH) guidelines (2005), covering aspects such as specificity, selectivity, linearity, accuracy, precision, Limit of Quantification (LOQ), and Limit of Detection (LOD).
Specificity/selectivity:
Selectivity is the ability of an analytical method to differentiate and measure the analyte in the presence of potential interfering substances in the sample. The evaluation of selectivity showed no significant response from interfering ingredients at the retention time of the analyte. The retention time of allopurinol was 1.2 min[20]. This method demonstrated high specificity in detecting the allopurinol analyte, even in the presence of other components in the formulation.
Linearity and range:
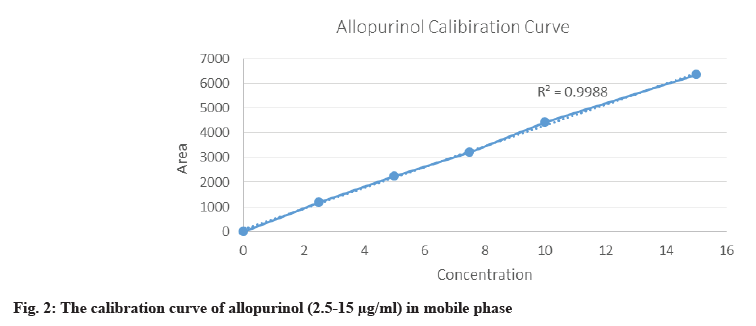
As per ICH guidelines, the linearity of the analytical method was demonstrated by its ability to produce test results directly proportional to the analyte concentration in samples within a specified range. Various aliquots of the standard drug solution were prepared from the stock solution and analyzed to assess the linearity of the proposed method[6]. The drug exhibited linearity within the concentration range of (2.5-15) μg/ml, with a correlation coefficient (r2≥0.9988).
Accuracy and precision:
The intraday accuracy and precision study were carried out by preparing a set of calibration standard curve samples and 10 quality control samples of each concentration (low (6 μg/ml), medium (12.5 μg/ ml), and high (20 μg/ml)) were analyzed in a day. In addition, interday accuracy and the precision study were carried out by preparing a set of calibration standard curve samples and 5 quality control samples of each concentration (low (6 μg/ml), medium (12.5 μg/ml), and high (20 μg/ml)) were analyzed in 3 d. The mean percentage accuracy, Standard Deviation (SD), and percentage Coefficient of Variation (% CV) were calculated for both intraday and interday validation.
Application of the proposed method for pharmaceutical formulation:
The assay test for various strengths of allopurinol tablets aimed to determine the precise quantity of the active ingredient. Each batch analysis involved the preparation of 5 calibration standards, 5 quality control samples, and 5 assay samples, all prepared for injection into the RP-HPLC method.
Results and Discussion
The most fundamental challenge in our study is to develop and validate different methods using various instruments while applying the same range of calibration standards for the curve (fig 2). The proposed HPLC method was validated in accordance with the ICH guidelines, assessing parameters such as specificity, selectivity, linearity, accuracy, precision, LOQ, LOD, and robustness[6]. The validated chromatography results analyzed by Ultra High Performance Liquid Chromatography (UHPLC) showed excellent linearity with a correlation coefficient (r2≥0.9988) in the concentration range of (2.5-15) μg/ml. Intraday and interday accuracy were between 10 %-110 %, with an intraday and interday % CV error of <10 %. The Lower LOD (LLOD) and the Lower LOQ (LLOQ) are shown in fig. 2. In addition, the method demonstrated high sensitivity and was applicable for the study. All validation parameters were conducted following ICH guidelines. The validation data confirmed that the HPLC method in aqueous solutions is sensitive, accurate, and precise. The intraday and interday precisions (% CV) were both <2 %, within the accepted range of ±10 %. The intraday and interday accuracy of allopurinol ranged from 103 %-110 %, which is within the accepted range of 90 %-110 % as shown in Table 1.
| Proposed methods | Paracetamol concentration (µg/ml) | Mean | Accuracy (%) |
SD | % CV |
|---|---|---|---|---|---|
| Intraday validation (accuracy and precision) | 6 | 6.594 | 109.9 | 0.0157 | 0.2377 |
| 12.5 | 12.9559 | 103.6475 | 0.0522 | 0.4031 | |
| 20 | 20.5974 | 102.9871 | 0.1044 | 0.5071 | |
| Interday validation (accuracy and precision) | 6 | 6.6243 | 110.4049 | 0.1914 | 2.8892 |
| 12.5 | 13.0355 | 104.2837 | 0.2803 | 2.1506 | |
| 20 | 20.7704 | 103.8522 | 0.4291 | 2.0658 |
Table 1: Summary of Accuracy and Precision Results of the Proposed HPLC Method
Furthermore, the chromatography technique demonstrates the LLOQ and LLOD as 4.09 μg/ml and 1.36 μg/ml, respectively, based on the following equations:
LLOD=3.3×SD/slope and LLOQ=10×SD/slope[6].
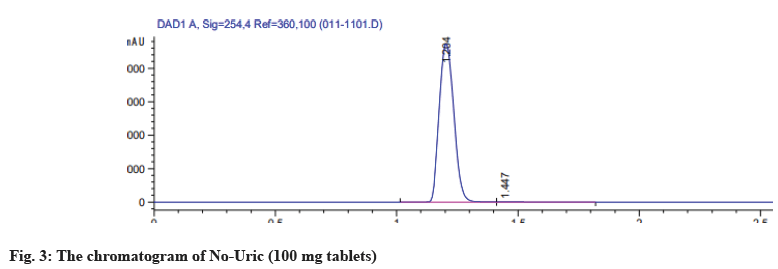
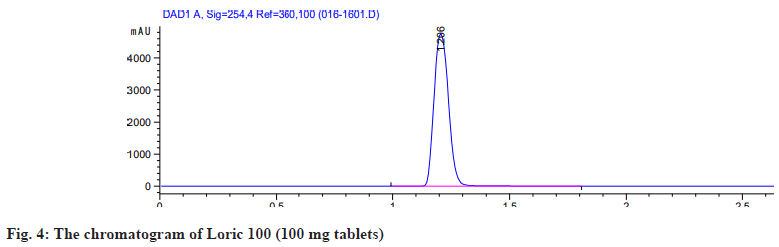
2 pharmaceutical formulation of allopurinol tablets, No-uric and Loric100 were analyzed by the HPLC method. The peak area of the sample solution was measured and the amount of allopurinol present in the tablet formulation was determined by the calibration curve. The assay results were summarized in Table 2. The percentage content of active ingredients in 2 allopurinol tablets showed acceptable values of 99.01 %-100.83 %, which comply with ICH acceptance criteria of (90 %-110 %)[28]. The chromatogram showed the separation of allopurinol (100 mg tablets) No-uric and Loric 100 both made to an injectable dilution of 50 μg/ml as shown in fig. 3 and fig. 4.
| Name of product | Strength (mg) | Test product concentration (µg/ml) | Average (of six samples) | Assay (%) | SD | % RSD |
|---|---|---|---|---|---|---|
| Loric 100 | 100 | 50 | 53.902544 | 53.902544 | 0.290021 | 0.538046 |
| No-Uric | 100 | 50 | 52.49989 | 52.49989 | 0.234119 | 0.445942 |
Table 2: Allopurinol Assay Tests were Analyzed by the HPLC
The present study utilized validated analytical techniques in compliance with ICH guidelines, ensuring accurate and sensitive quantification of allopurinol. Notably, the HPLC method demonstrated superior accuracy, precision, linearity, and sensitivity. These analytical approaches are well-suited for a variety of in vitro applications, including quality control and stability testing.
Authors’ contributions:
Abeer Ayidh Alghamdi, Aljawharah Mohammed Alanazi, Fawziah Harb Hommady, and Norah Aldeghaither have contributed to the development of the methodology and the drafting of the manuscript. Ibrahim Farh supervised all practical work, as well as revising and reviewing the manuscript. Sultan Alqahtani and Mahmoud Mansour proposed the research topic and experimental design.
Funding:
This work was supported by a grant from the King Abdullah International Research Center, National Guard Health Affairs, Riyadh, Saudi Arabia (Grant No: SP23R/166/06).
Conflict of interests:
The authors declared no conflict of interests.
References
- Pea F. Pharmacology of drugs for hyperuricemia. Mechanisms, kinetics and interactions. Contrib Nephrol 2005;147:35-46.
[Crossref] [Google Scholar] [PubMed]
- Reiter S, Simmonds HA, Zollner N, Braun SL, Knedel M. Demonstration of a combined deficiency of xanthine oxidase and aldehyde oxidase in xanthinuric patients not forming oxipurinol. Clinica Chimica Acta 1990;187(3):221-34.
[Crossref] [Google Scholar] [PubMed]
- Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity: Description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984;76(1):47-56.
[Crossref] [Google Scholar] [PubMed]
- Perez‐Ruiz F, Atxotegi J, Hernando I, Calabozo M, Nolla JM. Using serum urate levels to determine the period free of gouty symptoms after withdrawal of long‐term urate‐lowering therapy: A prospective study. Arthritis Rheum 2006;55(5):786-90.
[Crossref] [Google Scholar] [PubMed]
- Hande K, Reed E, Chabner B. Allopurinol kinetics. Clin Pharmacol Ther 1978;23(5):598-605.
[Crossref] [Google Scholar] [PubMed]
- Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med 2009;47(12):1673-706.
[Crossref] [Google Scholar] [PubMed]
- Sato M, Mamada H, Anzai N, Shirasaka Y, Nakanishi T, Tamai I. Renal secretion of uric acid by organic anion transporter 2 (OAT2/SLC22A7) in human. Biol Pharm Bull 2010;33(3):498-503.
[Crossref] [Google Scholar] [PubMed]
- Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: A retrospective analysis of administrative claims. Int J Rheumatol 2004;31(8):1575-81.
[Google Scholar] [PubMed]
- Mikuls TR, Saag KG. Gout treatment: What is evidence-based and how do we determine and promote optimized clinical care? Curr Rheumatol Rep 2005;7(3):242-9.
[Google Scholar] [PubMed]
- Tada H, Fujisaki A, Itoh K, Suzuki T. Facile and rapid high‐performance liquid chromatography method for simultaneous determination of allopurinol and oxypurinol in human serum. J Clin Pharm Ther 2003;28(3):229-34.
[Crossref] [Google Scholar] [PubMed]
- de Vries JX, Voss A, Ittensohn A, Walter-Sack I, Loffler W, Landthaler R, et al. Interaction of allopurinol and hydrochlorothiazide during prolonged oral administration of both drugs in normal subjects: II. Kinetics of allopurinol, oxipurinol, and hydrochlorothiazide. J Clin Invest 1994;72:1076-81.
- KojIma T, Nishina T, Kitamura M, Kamatani N, Nishioka K. Reversed-phase liquid-chromatographic determination of purine compounds in serum applied to studies of hypouricemia. Clin Chem 1986;32(2):287-90.
[Crossref] [Google Scholar] [PubMed]
- Reinders MK, Nijdam LC, van Roon EN, Movig KL, Tim LT, van de Laar MA, et al. A simple method for quantification of allopurinol and oxipurinol in human serum by high-performance liquid chromatography with UV-detection. J Pharm Biomed Anal 2007;45(2):312-7.
[Crossref] [Google Scholar] [PubMed]
- Boulieu R, Bory C, Baltassat P, Gonnet C. Simultaneous determination of allopurinol, oxipurinol, hypoxanthine and xanthine in biological fluids by high-performance liquid chromatography. J Chromatogr 1984;307:469-74.
[Crossref] [Google Scholar] [PubMed]
- Nissen P. Simultaneous determination of allopurinol, oxipurinol and uric acid in human plasma by high-performance liquid chromatography. J Chromatogr 1982;228:382-6.
[Crossref] [Google Scholar] [PubMed]
- Breithaupt H, Goebel G. Determination of allopurinol and oxipurinol in biological fluids by high-performance liquid chromatography. J Chromatogr 1981;226(1):237-42.
[Crossref] [Google Scholar] [PubMed]
- Wung WE, Howell SB. Simultaneous liquid chromatography of 5-fluorouracil, uridine, hypoxanthine, xanthine, uric acid, allopurinol, and oxipurinol in plasma. Clin Chem 1980;26(12):1704-8.
[Crossref] [Google Scholar] [PubMed]
- Kramer WG, Feldman S. High-performance liquid chromatographic assay for allopurinol and oxipurinol in human plasma. Chromatogr 1979;162(1):94-7.
[Crossref] [Google Scholar] [PubMed]
- Dastiagiriamma D, Kistayya C, Sowjanya HM, Hemalatha K. Simultaneous estimation of lesinurad and allopurinol by using reverse phase high performance liquid chromatography in API and marketed formulation. Int J Med Pharm Sci 2018;3(7)1-6.
- Khader S, Begum A, Ramakrishna D. Development and validation of reverse phase HPLC method for simultaneous estimation of allopurinol and lesinurad in its API and pharmaceutical dosage form. Int J Appl Pharm Sci Res 2019;4(4):50-7.
- Khayoon WS, Al-Abaichy MQ, Jasim M, Al-Hamadany MA. Spectrophotometric determination of allopurinol in tablet formulation. J Phys Sci 2008;19(2):23-30.
- Hassib ST, Safwat HM, El-Bagry RI. Spectrophotometric determination of some anti-inflammatory agents using N-bromosuccinimide. Analyst 1986;111(1):45-8.
[Crossref] [Google Scholar] [PubMed]
- Pérez-Ruiz T, Martı́nez-Lozano C, Tomás V, Galera R. Development of a capillary electrophoresis method for the determination of allopurinol and its active metabolite oxypurinol. J Chromatogr B Analyt Technol Biomed Life Sci 2003;798(2):303-8.
[Crossref] [Google Scholar] [PubMed]
- Sun X, Cao W, Bai X, Yang X, Wang E. Determination of allopurinol and its active metabolite oxypurinol by capillary electrophoresis with end-column amperometric detection. Anal Chim Acta 2001;442(1):121-8.
- Zen JM, Chen PY, Kumar AS. Flow injection analysis of allopurinol by enzymeless approach at glassy carbon electrodes. Electroanalysis 2002;14(10):645-9.
- Chatten LG, Boyce M, Moskalyk RE, Pons BS, Madan DK. Determination of allopurinol in tablets by differential-pulse polarography. Analyst 1981;106(1260):365-8.
[Crossref] [Google Scholar] [PubMed]
- Bakr MH, Hassan AN. Spectrophotometric determination of allopurinol by oxidative coupling reaction using 2-nitrophenol reagent in the presence of N-bromosuccinimide. Pak J Med Sci 2022;16(6):814.
- ICH. Q2(R1): Validation of analytical procedures: Text and methodology. In International Conference on Harmonization, Geneva; 2005.