- *Corresponding Author:
- Yunfei Jiang
Department of Emergency Medicine,
Nanjing Drum Tower Hospital,
The Affiliated Hospital of Nanjing University Medical School,
Nanjing 210008,
China
E-mail: jiangyunfei@njglyy.com
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “103-110” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Multidrug resistance Klebsiella pneumoniae infection is an essential factor threatening the life of patients with severe infection. Polymyxin B is widely used in treating multidrug resistance Klebsiella pneumoniae, but few studies on its efficacy and safety. In our study, 205 patients with multidrug resistance Klebsiella pneumoniae infection were admitted to our hospital from January 2017 to December 2020, treated with polymyxin B for antibacterial and selected as the subjects. These patients were divided into two therapy groups: A monotherapy group (polymyxin B) and a combined therapy group (polymyxin B-tigecycline). The efficacy and safety of the two groups were analyzed. Also, the risk factors were analyzed. The changes of cystatin C, beta 2-microglobulin and microalbuminuria in the two groups showed an upward trend during the treatment. The descent degree of procalcitonin, C-reaction protein and white blood cell count in the polymyxin B-tigecycline group was higher than that in the polymyxin B group. The two groups had a similar effective rate, while acute kidney injury incidence in the polymyxin B-tigecycline group was higher than in the polymyxin B group. Blood infection, hypertension, high dose of tigecycline were risk factors for acute kidney injury. It was the first time to report that hypertension is an independent risk factor of acute kidney injury when polymyxin B combined with tigecycline is used to treat multidrug resistance Klebsiella pneumoniae infection. Patients with hypertension infected with multidrug resistance Klebsiella pneumonia should avoid accepting the combination therapy of polymyxin B and tigecycline.
Keywords
Polymyxin B, infection, Klebsiella pneumoniae, acute kidney injury
Klebsiella pneumoniae is the clinically significant opportunistic pathogen, also the common pathogenic bacteria causing the nosocomial infection [1,2]. It can quickly form the biofilm and readily colonize, easily mutate and have multi-drug resistance [3]. Klebsiella pneumoniae, which is not the common customized bacteria in the human body, generally can be isolated from the skin, nasal mucosa, throat, faeces and other parts of ordinary people [1,3]. In patients’ Klebsiella pneumoniae infected rate is higher than 50 %, especially the patients with trauma, mechanical ventilation, catheter, surgery and severe burns [3,4]. According to the China Antimicrobial Resistance Surveillance System statistics, the incidence of infections caused by Klebsiella pneumoniae is increasing year by year, which is No. 2 of the number of clinically isolated strains in China in 2020 and the leading cause of patient deaths [5]. For the infection caused by Multidrug Resistance Klebsiella pneumoniae (MDR-KP), the primary clinical therapy protocol should combine various antimicrobials [6,7].
Polymyxin, a narrow-spectrum antibiotic, has good activity against most Gram-Negative Bacteria (GNB) and natural resistance to Gram-Positive Bacteria (GPB), fungi, anaerobic bacteria, some gram-negative cocci (Neisseria gonorrhoeae, Neisseria meningitidis) and parasites, etc., And it is naturally sensitive to Pseudomonas aeruginosa and Acinetobacter baumannii [8,9]. It can also effectively resist Haemophilus influenzae, Escherichia coli, Salmonella, Shigella, Klebsiella pneumoniae, Legionella pneumophila, Citrobacter and Bordetella pertussis. Polymyxin can kill bacteria rapidly by destroying the integrity of the cell membrane [10]. It was widely used to treat the GNB clinically, but the narrow antibacterial spectrum, apparent toxic and side effects drive it into replacement gradually [10,11]. With the increasing infection rate of MDR-GNB globally, polymyxin as the last line of defence for treating GNB infection, people began to value it again [12]. At present, polymyxin B and polymyxin E are two kinds of polymyxin used in clinical. Compared with polymyxin E, polymyxin B has an excellent pharmacokinetic effect [10,13]. However, due to limited drug resources, it relatively lacks clinical data and clinical treatment experience for Polymyxin B, especially with tigecycline [14].
Tigecycline, a low resistance rate to Klebsiella pneumoniae, is often combined with other drugs for antibacterial therapy, including carbapenems, cephalosporins, penicillins and bacillosporin [15,16]. This study aims to investigate the efficacy and safety of polymyxin B and tigecycline combined with MDRKP by retrospective analysis to provide evidence for doctors’ clinical applications.
Materials and Methods
Study design:
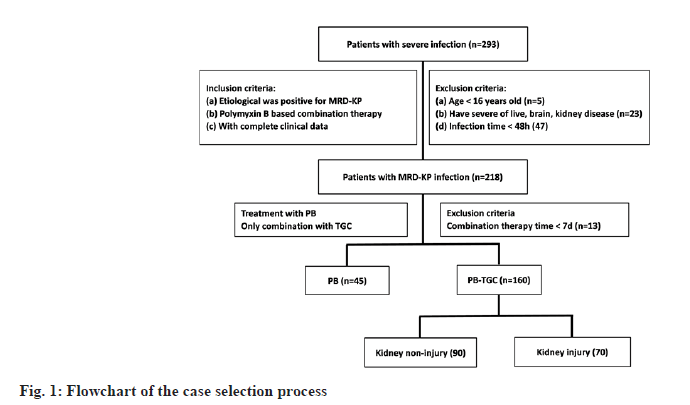
In this study, 293 patients were selected as the subjects. They had a severe infection and were admitted to our hospital from January 2017 to December 2020. Collected patients’ information based on the medical record system in the hospital, including the basic information of the patient (age, gender, clinical diagnosis, inpatient department, etc.,); the basic information of medication (dosage, frequency, time, combined use of antibiotics); the situation and results of bacterial culture; patient infection index (pre-and post-infection signs, White Blood Cell Count (WBC), C-Reaction Protein (CRP), Procalcitonin (PCT) value, etc.,) and the early kidney function index of the patient (Cystatin C (Cyc-C), microalbuminuria (mALB), beta2-Microglobulin (β2- MG)). In addition, this study did not directly interfere with the patients and showed the patients, names. In order to improve the accuracy of the analysis, we had strictly controlled the inclusion criteria so that only patients with purity MDR-KP infection could be included in this study. The study would exclude patients with the following conditions: Use other antibiotics except for polymyxin B and tigecycline; <16 y old; had severe liver, brain, kidney diseases; infection time was less than 48 h; combined treatment time was less than 7 d. Assessed the severity of diseases by using the Acute Physiology and Chronic Health Evaluation (APACHE) II scores and Acute Kidney Injury (AKI) risk prediction scores calculated when MDR-KP attacks [17].
Data analysis:
As shown in fig. 1, according to include and exclude conditions, 205 patients were finally selected as the research object. According to antibiotics, these patients were divided into two groups (Polymyxin B (PB), n=45 and polymyxin B-tigecycline (PB-TGC), n=160); according to AKI occurrence, 160 patients in the PB-TGC group were divided into two groups (kidney injury group, n=70 and kidney non-injury group, n=90). It is worth mentioning that the patients included in this study did not die during the treatment period and because of the patients' active discharge, we could not track and count the recovery of kidney function after treatment.
Microbiological assessment and definition of terms:
.The identification and antimicrobial susceptibility of Klebsiella pneumoniae were determined by using the Vitek 2 system (bioMérieux, Marcy-l'Etoile, France). MDR-KP onset was defined as the collection date of the first positive biological simple culture. According to the guidelines of the Clinical and Laboratory Standards Institute standards (2015), the definition of carbapenem-nonsusceptibility was a Minimum Inhibitory Concentration (MIC) of ≥1 mg/l for ertapenem or ≥2 mg/l for imipenem or meropenem [18]. The US Food and Drug Administration (FDA) breakpoints were used to judge tigecycline and polymyxin B susceptibilities [19]. The in vitro susceptibility test results showed that the pathogenic bacteria isolated from the patients were sensitive to polymyxin B and tigecycline. The MIC of tigecycline and polymyxin B was determined by using standard broth microdilution tests with fresh (<12 h) Mueller–Hinton II Broth (cation-adjusted; Solarbio Science and Technology Ltd., Beijing, People’s Republic of China). According to the using dose, the dose of tigecycline was classified into low-dose (loading dose 100 mg, maintenance dose 50 mg/12 h) and high-dose (loading dose ≥100 mg, maintenance dose >50 mg/12 h) [20] and the dose of polymyxin B was classified into low-dose (<1.5 mg/kg) and high-dose (>1.5 mg/kg) [21]. The efficacy of antibacterial therapy was defined by the patient's negative etiological examination 3 d after discontinuation.
Statistical analysis:
In this study, statistical analysis used Statistical Package for the Social Sciences (SPSS) 24.0. Mean±Standard Deviation (SD) expressed the measurement data and Percentage (%) expressed the count data. Chi-square (χ2) was used for qualitative data andalphat-test was used for quantitative data. Variables with p<0.05 in the univariate analysis were used in binary logistic regression. Multivariate analysis was used to identify independent predictors. The correlation analysis between variables, 95 % Confidence Interval (CI), used logistic regression and the R and p value expressed the results. In all analyses, p<0.05 was considered significant.
Results and Discussion
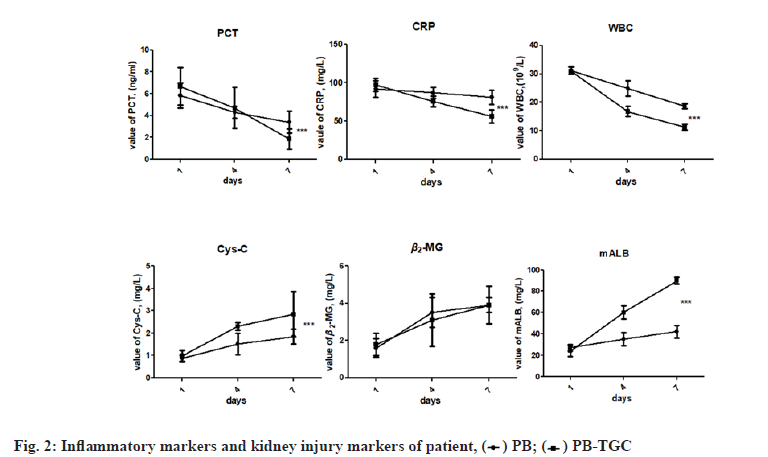
In this study, 205 patients were finally included, wherein the minimum age was 22 y old; the maximum age was 92 y old; the average age was 60.77±13.22 y old, of which 142 were male (69.2 %). As shown in Table 1, the patients with pulmonary infection account for the majority (103/205, 50.24 %), followed by blood infection (15/205, 26.82 %). In terms of underlying diseases, hypertension accounts for the majority (104/205, 50.7 %) and then diabetes (58/205, 27.8 %). The case distribution in inpatient departments was as follows: 66 cases in the intensive care unit, 65 cases in pulmonary and critical care medicine, 11 cases in general medicine department, 51 cases in emergency center and 12 cases in neurosurgery department. Compared to the demographic and characteristics in the two groups, three projects showed significant differences (p<0.001), namely, skin infections, urinary infections and AKI scores. The incidence of AKI in the PB-TGC group (70/160, 43.8 %) was higher than PB group (8/45, 17.8 %). Two groups had similar antibacterial efficacy (69.1 % vs. 68.8 %, p=0.913). As shown in fig. 2, in the period of antimicrobial therapy, the inflammatory factors of the two groups showed a downtrend, while the descent degree of the PB-TGC group was significantly higher than that of the PB group. The kidney injury-related factors in the early stage of the two groups were within the standard limit, while with the ongoing antimicrobial therapy, the value of Cys-C and mALB in the PB-TGC group was significantly higher than that of the PB group (p<0.05).
| Demographic | PB (n=45) | PB-TGC (n=160) | p value |
|---|---|---|---|
| Male, n (%) | 30 (66.7) | 112 (70) | 0.217 |
| Female, n (%) | 15 (33.3) | 48 (30) | |
| Age, years, mean±SD | 59.60±16.97 | 61.10±12.17 | 0.944 |
| APACHE II, score, mean±SD | 24.68±8.05 | 23.57±9.196 | 0.547 |
| Period of treatment, d, mean±SD | 11.89±1.22 | 9.85±1.21 | 0.205 |
| Infection sitea, n (%) | |||
| Pulmonary | 23 (51.1) | 78 (48.8) | 0.017 |
| Blood | 15 (33.3) | 40 (25) | 0.44 |
| Abdominal cavity | 6 (13.3) | 23 (14.4) | 0.418 |
| Skin | 1 (2.2) | 16 (10.0) | <0.001 |
| Urinary | 3 (6.7) | 3 (1.9) | <0.001 |
| Underlying diseasesb, n (%) | |||
| Hypertension | 29 (64.4) | 75 (46.9) | 0.494 |
| Diabetes | 15 (33.3) | 43 (26.8) | 0.361 |
| Atrial fibrillation | 14 (31.1) | 38 (23.7) | 0.532 |
| Rheumatoid arthritis | 2 (4.4) | 2 (1.3) | |
| Prostatic hyperplasia | 2 (4.4) | 2 (1.3) | |
| Pancreatitis | 0 (0) | 2 (1.3) | |
| AKI score, n (%) | |||
| 1 grade | 8 (17.8) | 35 (21.8) | <0.001 |
| 2 grade | 0 (0) | 16 (10.0) | |
| 3 grade | 0 (0) | 19 (11.9) | |
| Efficiency % | 69.1 | 68.75 | 0.913 |
Note: Data is expressed as numbers (%) unless otherwise stated; apatients with multiple-site infections will be counted separately; bpatients with multiple underlying diseases will be counted separately, PB: Polymyxin B; PB-TGC: Polymyxin B-Tigecycline
Table 1: Clinical and Demographic Characteristics of Patients Caused by Multi-Drug Resistance Klebsiella Pneumonia
In the light of study design conditions, 205 patients were divided into two groups, the PB group (45/205, 22.0 %) and the PB-TGC group (160/205, 78.0 %). The results of the univariate analysis indicated that the factors independently associated with a higher risk of AKI were as follows: Male, age, APACHE II score, blood infection, hypertension, CRP, WBC, mALB and higher dose of PB and TGC. The results of multivariate analysis indicate that the factors independently associated with a higher risk of AKI were as follows: Blood infection (OR=3.527, 95 % CI=1.153-9.201, p=0.026), hypertension (OR=6.727, 95 % CI=1.446-27.206, p=0.014), WBC (OR=3.922, 95 % CI=1.064-14.464, p=0.014), dose of TGC <0.2 g/d (OR=0.096, 95 % CI=0.032-0.288, p=0.001) and dose of TGC >0.2 g/d (OR=0.023, 95 % CI=0.006-0.088, p=0.001), as shown in Table 2.
| Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| PB (n=45) | PB-TGC (n=160) | p value | Sig. | EXP (B) | 95 % CI for EXP (B) | ||
| Lower | Upper | ||||||
| Demographic | |||||||
| Male, n (%) | 30 (66.7) | 7 (70) | 0.046 | ||||
| Age, years, mean±SD | 65.68±18.03 | 61.10±12.17 | 0.015 | ||||
| APACHE II, score, mean±SD | 24.68±8.05 | 23.57±9.196 | 0.012 | ||||
| Period of treatment, d, mean±SD | 11.89±1.22 | 9.85±1.21 | 0.187 | ||||
| Infection sitea | |||||||
| Pulmonary, n (%) | 23 (51.1) | 78 (48.8) | 0.209 | ||||
| Blood, n (%) | 15 (33.3) | 40 (25) | 0.005 | 0.026 | 3.527 | 1.153 | 9.201 |
| Seroperitoneum, n (%) | 6 (13.3) | 23 (14.4) | 0.214 | ||||
| Skin wound, n (%) | 1 (2.2) | 16 (10.0) | 0.226 | ||||
| Urine, n (%) | 3 (6.7) | 3 (1.9) | 0.606 | ||||
| Underlying diseasesb | |||||||
| Hypertension, n (%) | 29 (64.4) | 75 (46.9) | <0.001 | 0.014 | 6.272 | 1.446 | 27.206 |
| Diabetes, n (%) | 15 (33.3) | 43 (26.8) | 0.339 | ||||
| Atrial fibrillation, n (%) | 14 (31.1) | 38 (23.7) | 0.437 | ||||
| Rheumatoid arthritis, n (%) | 2 (4.4) | 2 (1.3) | 0.094 | ||||
| Prostatic hyperplasia, n (%) | 2 (4.4) | 2 (1.3) | 0.976 | ||||
| Pancreatitis, n (%) | 0 (0) | 2 (1.3) | 0.172 | ||||
| Inflammatory markers | |||||||
| PCT, >0.5 ng/ml, n (%) | 20 (44.4) | 33 | 0.955 | ||||
| CRP, >8 mg/l, n (%) | 27 (60.0) | 64 | <0.001 | ||||
| WBC, >9.5×109/l, n (%) | 18 (40.0) | 37 | 0.004 | 0.040 | 3.922 | 1.064 | 14.464 |
| Renal injury markers | |||||||
| Cys-C, >1.03 mg/l, n (%) | 9 (20.0) | 23 | 0.123 | ||||
| β2-MC, >2.4 mg/l, n (%) | 7 (15.6) | 12 | 0.940 | ||||
| mALB, >30 mg/l, n (%) | 12 (26.7) | 30 | 0.001 | ||||
| Polymyxin B | |||||||
| <1.5 mg/kg/d | 11 (24.4) | 87 (54.3) | 0.742 | ||||
| ≥1.5 mg/kg/d | 34 (75.6) | 73 (45.6) | <0.001 | ||||
| Tigecycline | |||||||
| <0.2 g/d | 0 (0) | 96 (60.0) | <0.001 | 0.001 | 0.096 | 0.032 | 0.288 |
| ≥0.2 g/d | 0 (0) | 64 (40.0) | 0.001 | 0.001 | 0.023 | 0.006 | 0.088 |
Note: Data is expressed as mean±SD unless otherwise stated; apatients with multiple-site infections will be counted separately; bpatients with multiple underlying diseases will be counted separately, PB: Polymyxin B; PB-TGC: Polymyxin B-Tigecycline
Table 2: Analysis of Risk Factors for Aki All Patients with MDR-KP
In the light of study design conditions, 160 patients in the PB-TGC group were divided into two groups, the kidney injury group (70/160, 43.8 %) and the kidney non-injury group (90/160, 56.2 %). As shown in Table 3, the results of the univariate analysis indicated that the factors independently associated with a higher risk of AKI were as follows: APACHE II, period of treatment, blood infection, abdominal cavity infection, skin infection, hypertension, diabetes, atrial fibrillation, WBC, β2-MG, mALB and dose of PB and TGC. The results of multivariate analysis indicate that the factors independently associated with a higher risk of AKI were as follows: APACHE II (OR=1.162, 95 % CI=0.948-1.425, p<0.001), period of treatment (OR=1.047, 95 % CI=0.837-1.413, p<0.001), blood infection (OR=0.899, 95 % CI=0.283-2.858, p<0.001), hypertension (OR=8,732, 95 % CI=2.264-3.366, p=0.007), Cys-C (OR=0.942, 95 % CI=0.671-1.323, p<0.001), β2-MG (OR=1.048, 95 % CI=0.801-1.370, p<0.001) and dose of PB ≥1.5 mg/kg/d (OR=0.801, 95 % CI=0.587-1.029, p<0.001) and dose of TGC ≥0.2 g/d (OR=0.854, 95 % CI=0.654-1.115, p<0.001).
| Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| Injury (n=70) | Non-injury (n=90) | p value | Sig. | EXP (B) | 95 % CI for EXP (B) | ||
|
Lower | Upper | |||||
| Demographic | |||||||
| Male, n (%) | 48 (68.2) | 61 (67.8) | 0.838 | ||||
| Age, years, mean±SD | 66.33±13.32 | 67.42±12.73 | 0.589 | ||||
| APACHE II, mean±SD | 27.45±10.16 | 25.66±6.79 | <0.001 | <0.001 | 1.162 | 0.948 | 1.425 |
| Period of treatment, d, mean±SD | 12.63±1.51 | 7.07±0.91 | <0.001 | <0.001 | 1.047 | 0.837 | 1.413 |
| Infection sitea | |||||||
| Pulmonary, n (%) | 34 (48.8) | 44 (48.8) | 0.160 | ||||
| Blood, n (%) | 18 (25.0) | 22 (24.4) | <0.001 | <0.001 | 0.899 | 0.283 | 2.858 |
| Abdominal cavity, n (%) | 10 (14.8) | 13 (14.4) | <0.001 | ||||
| Skin, n (%) | 0 (0) | 9 (100.0) | |||||
| Urinary, n (%) | 0 (0) | 3 (100.0) | |||||
| Underlying diseasesb | |||||||
| Hypertension, n (%) | 33 (46.9) | 42 (46.7) | <0.001 | 0.007 | 8.732 | 2.264 | 3.366 |
| Diabetes, n (%) | 19 (26.9) | 24 (26.7) | <0.001 | ||||
| Atrial fibrillation, n (%) | 17 (23.7) | 21 (23.3) | <0.001 | ||||
| Rheumatoid arthritis, n (%) | 0 (0) | 2 (100) | |||||
| Prostatic hyperplasia, n (%) | 0 (0) | 2 (100) | |||||
| Pancreatitis, n (%) | 0 (0) | 2 (100) | |||||
| Inflammatory markers | |||||||
| PCT, >0.5 ng/ml, n (%) | 20 (28.6) | 25 (27.8) | 0.191 | ||||
| CRP, >8 mg/l, n (%) | 27 (16.8) | 37 (41.1) | 0.015 | ||||
| WBC, >9.5×109/l, n (%) | 18 (11.3) | 19 (21.1) | <0.001 | ||||
| Renal injury markers | |||||||
| Cys-C, >1.03 mg/l, n (%) | 9 (5.6) | 14 (15.6) | 0.003 | <0.001 | 0.942 | 0.671 | 1.323 |
| β2-MC, >2.4 mg/l, n (%) | 7 (4.3) | 5 (5.6) | <0.001 | <0.001 | 1.048 | 0.801 | 1.370 |
| mALB, >30 mg/l, n (%) | 12 (7.5) | 18 (20) | <0.001 | ||||
| Dose of polymyxin B | |||||||
| <1.5 mg/kg/d | 30 (42.8) | 57 (63.3) | 0.111 | ||||
| ≥1.5 mg/kg/d | 40 (57.1) | 33 (36.7) | 0.013 | <0.001 | 0.801 | 0.587 | 1.092 |
| Dose of tigecycline | |||||||
| <0.2 g/d | 34 (48.5) | 62 (68.9) | 0.031 | ||||
| ≥0.2 g/d | 36 (51.4) | 28 (31.1) | <0.001 | <0.001 | 0.854 | 0.654 | 1.115 |
Note: Data is expressed as mean±SD unless otherwise stated; apatients with multiple-site infections will be counted separately; bpatients with multiple underlying diseases will be counted separately
Table 3: Analysis of Risk Factors for Aki in PB-TGC Group with MDR-KP Analysis of Risk Factors for Aki in PB-TGC Group with MDR-KP
Currently, polymyxin B is widely used in various severe infections such as pneumonia, urinary tract infection and septicemia caused by Pseudomonas aeruginosa and Acinetobacter baumannii [10,13,14]. However, most related studies are carried out in vitro and relevant clinical research data is small. Thus the rationality and safety evaluation of combined medication is limited [10,14,22]. Another reason is that the clinical practice is usually confused with various drugs for antibacterial treatment, so interaction between these drugs has not been known. Therefore, based on the limited number of patients, it is meaningful to investigate the efficacy of the combined application of polymyxin B and tigecycline to treat MDR-KP by controlling variables. Previous studies have shown that the adverse reactions of polymyxin B are nephrotoxicity and neurotoxicity and other rare side effects include allergic reaction, skin itching and mild irritative cough when inhaling the preparation [11,14]. It is interesting that, except for the relatively high incidence of AKI, the incidence of other adverse reactions was extremely low, about 1 %. Thus the statistics of those adverse reactions were not performed in this study. For the highest incidence of adverse reactions, both in the PB group and PB-TGC group, AKI appeared around the fourth day of the beginning of antimicrobial treatment. In order to study the high incidence of AKI, we evaluated patients' characteristics and treatments. Furthermore, we identified the risk factors for AKI through the analysis of intergroup (PB group vs. PB-TGC group) and intragroup (PB-TGC group, kidney injury group vs. kidney non-injury group).
As shown in Table 2 and Table 3, the results indicated that blood infection, hypertension and a high dose of TGC ≥0.2 g/d were the high-risk factors for AKI. Severe blood infection often leads to multiple organ failure and shock, with a high mortality rate [23]. Septic AKI occurs between 15 % and 20 % of all intensive care unit admissions and its mortality ranges from 20 % to 60 %. During the study period, we observed that patients usually had pulmonary infections before blood infection (10/78, 12.8 %) and the probability of blood infection was positively related to the treatment time. For patients whose infection is not resolved by conventional doses of tigecycline, an increased dose is often used, which is an approach that has also been recommended in a recent consensus statement [20]. There were no reports of kidney injury induced by tigecycline [19,20]. However, our study results indicated that doses of tigecycline ≥0.2 g/d were the high-risk factors for AKI. This might be related to the following facts: tigecycline and polymyxin B were both excreted through the kidney, wherein tigecycline was about 33 % [24,25] and polymyxin B was about 60 % [26], the combination of tigecycline and polymyxin B increased the kidney burden of drug metabolism and caused the increased incidence of kidney injury.
It is generally accepted that hypertension is a risk factor for kidney injury [27,28]. The damage of hypertension to the kidney is not transient but persistent and irreparable [29]. Furthermore, the kidney itself has a powerful compensatory capacity and under normal circumstances, just 30 % of kidney function can maintain the metabolism demand of the human body. Therefore, kidney injury in the early stage generally does not have clinical symptoms. When the clinical symptoms of kidney injury appeared, the degree of kidney injury had reached more than 70 %. In order to avoid the occurrence of the above situation, we selected Cys-C, β2-MG, mALB as the markers of kidney injury in the early-stage [30]. These indicators were within the standard limit before patients were included in the study to accept antimicrobial therapy. With the ongoing therapy, their values gradually increased, indicating that Cys-C, β2-MG and mALB can be used to diagnose kidney injury. We also observed that the decreasing trend of inflammatory related factors in PB-TGC group was more significant than that in PB group (p<0.001). The above results indicated that for MDR-KP, the therapeutic efficacy of combination application of polymyxin B and tigecycline was better than that of polymyxin B alone, but the relatively higher incidence of kidney injury is worthwhile concerning of clinicians, especially for patients with hypertension. Unfortunately, we do not know why hypertension is an independent risk factor for AKI when polymyxin B and tigecycline are combined for anti-MDR-KP therapy. This issue will be further studied in the future.
The present study is associated with some limitations. Firstly, the sample size of each group in this study is relatively small. Secondly, only acute kidney injury is analyzed and the patients are not followed up for an extended period. Finally, the clinical effect and microbiological subgroup analysis of different combined treatment regimens are not performed in different bacterial groups.
To sum up, we analyzed the efficacy of polymyxin B combined with tigecycline to treat MDR-KP infection in this study. It was found that the combination of polymyxin B and tigecycline in the treatment of MDR-KP infection had a higher incidence of AKI. Through analysis of intergroup and intragroup, blood infection, hypertension and high dose of tigecycline were higher risk factors for acute kidney injury. It was the first time to report that hypertension was an independent risk factor of kidney injury when the combination of polymyxin B and tigecycline was used to treat MDR-KP infection. Therefore, we suggest that people with hypertension who are infected with MDR-KP should avoid using PB-TGC for antimicrobial treatment. This study provided a valuable clinical experience for polymyxin B and tigecycline combination therapy and provided clinical support and basis for patients with MDR-KP infection when choosing appropriate antimicrobial treatment.
Authors’ contributions:
Xiao Du and Hanwen Tong have contributed equally to this work. Yunfei Jiang and Binxia Shao are considered as co-corresponding authors.
Funding:
This work was supported by Nanjing Medical Center for Clinical Pharmacy.
Conflict of interests:
The authors declare that no conflict of interest is associated with this work.
References
- Rodríguez-Medina N, Barrios-Camacho H, Duran-Bedolla J, Garza-Ramos U. Klebsiella variicola: An emerging pathogen in humans. Emerg Microbes Infect 2019;8(1):973-88.
[Crossref] [Google Scholar] [Pub Med]
- Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect 2016;22:S9-14.
[Crossref] [Google Scholar] [Pub Med]
- Sharma S, Mohan H, Sharma S, Chhibber S. A comparative study of induction of pneumonia in mice with planktonic and biofilm cells of Klebsiella pneumoniae. Microbiol Immunol 2011;55(5):295-303.
[CrossRef] [Google Scholar] [Pub Med]
- Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect 2015;70(6):592-9.
[Crossref] [Google Scholar] [Pub Med]
- Zheng YG, Hu F, Zhu DM. CHINET surveillance of bacterial resistance in secondary care hospitals across China: Report of results in 2019. Chin J Infect Chemother 2020;20(6):9.
- Bailey KL, Kalil AC. Ventilator-associated pneumonia (VAP) with multidrug-resistant (MDR) pathogens: Optimal treatment? Curr Infect Dis Rep 2015;17(8):1-6.
[Crossref] [Google Scholar] [Pub Med]
- Rigatto MH, Ribeiro VB, Konzen D, Zavascki AP. Comparison of polymyxin B with other antimicrobials in the treatment of ventilator-associated pneumonia and tracheobronchitis caused by Pseudomonas aeruginosaorAcinetobacter baumannii. Infection 2013;41(2):321-8.
[Crossref] [Google Scholar] [Pub Med]
- Krishnamurthy M, Lemmon MM, Falcinelli EM, Sandy RA, Dootz JN, Mott TM, et al. Enhancing the antibacterial activity of polymyxins using a nonantibiotic drug. Infect Drug Resist 2019;12:1393.
[Crossref] [Google Scholar] [Pub Med]
- Zhang R, Shen Y, Walsh TR, Wang Y, Hu F. Use of polymyxins in Chinese hospitals. Lancet Infect Dis 2020;20(10):1125-6.
[Crossref] [Google Scholar] [Pub Med]
- Soman R, Bakthavatchalam YD, Nadarajan A, Dwarakanathan HT, Venkatasubramanian R, Veeraraghavan B. Is it time to move away from polymyxins? Evidence and alternatives. Eur J Clin Microbiol Infect Dis 2021;40(3):461-75.
[Crossref] [Google Scholar] [Pub Med]
- Falagas ME, Kasiakou SK, Tsiodras S, Michalopoulos A. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: A review of the recent literature. Clin Med Res 2006;4(2):138-46.
[Crossref] [Google Scholar] [Pub Med]
- Siwakoti S, Subedi A, Sharma A, Baral R, Bhattarai NR, Khanal B. Incidence and outcomes of multidrug-resistant gram-negative bacteria infections in intensive care unit from Nepal-A prospective cohort study. Antimicrob Resist Infect Control 2018;7(1):1-8.
[Crossref] [Google Scholar] [Pub Med]
- Glascott EL. Polymyxin B or polymyxin E: Does it really matter? J Pharm Pract Res 2018;48(5):492-4.
- Chen H, Guo X, Xie D, Dong X, Niu J, Chen G. A clinical study on the use of intraventricular Polymyxin B supplemented by continuous external ventricular drainage in the treatment of drug-resistant gram-negative bacilli intracranial infection. Infect Drug Resist 2020;13:2963.
[Crossref] [Google Scholar] [Pub Med]
- Townsend ML, Pound MW, Drew RH. Potential role of tigecycline in the treatment of community-acquired bacterial pneumonia. Infect Drug Resist 2011;4:77.
[Crossref] [Google Scholar] [Pub Med]
- Abdallah M, Alsaleh H. A review of safety and effectiveness of intravenous and Intraventricular tigecycline in healthcare-associated Acinetobacter baumannii meningitis and ventriculitis. Curr Treat Options Infect Dis 2019;11(4):331-43.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med 1985;13(10):818-29.
- Lalitha MK. Manual on antimicrobial susceptibility testing. Performance standards for antimicrobial testing: Twelth Informational Supplement 2004;56238:454-6.
- Greer ND. Tigecycline (Tygacil): The first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent) 2006;19(2):155-61.
- Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 2014;44(1):1-7.
[Crossref] [Google Scholar] [Pub Med]
- Yu X, Pan J, Zhou Z, Wen X, Dai Y, Lin G, et al. TDM-guided medication of polymyxin B in a patient with CRKP-induced bloodstream infection: A case report. Eur J Clin Microbiol Infect Dis 2021;40(1):201-4.
[Crossref] [Google Scholar] [Pub Med]
- Wilhelm CM, Nunes LD, Martins AF, Barth AL. In vitro antimicrobial activity of imipenem plus amikacin or polymyxin B against carbapenem-resistant Pseudomonas aeruginosa isolates. Diagn Microbiol Infect Dis 2018;92(2):152-4.
[Crossref] [Google Scholar] [Pub Med]
- Imai K, Ishibashi N, Kodana M, Tarumoto N, Sakai J, Kawamura T, et al. Clinical characteristics in blood stream infections caused by Klebsiella pneumoniae, Klebsiella variicola and Klebsiella quasipneumoniae: A comparative study, Japan, 2014–2017. BMC Infect Dis 2019;19(1):1-0.
[Crossref] [Google Scholar] [Pub Med]
- Stein GE, Babinchak T. Tigecycline: An update. Diagn Microbiol Infect Dis 2013;75(4):331-6.
[Crossref] [Google Scholar] [Pub Med]
- Kasbekar N. Tigecycline: A new glycylcycline antimicrobial agent. Am J Health Syst Pharm 2006;63(13):1235-43.
[Crossref] [Google Scholar] [Pub Med]
- Nation RL, Forrest A. Clinical pharmacokinetics, pharmacodynamics and toxicodynamics of polymyxins: Implications for therapeutic use. Adv Exp Med Biol 2019;145:219-49.
[Crossref] [Google Scholar] [Pub Med]
- de Menezes Neves PD, Reichert BV, Bridi RA, Yu L, Dias CB, Pinheiro RB, et al. A typical presentation of acute post-infectious glomerulonephritis in patients with sickle cell disease: Report of two cases. BMC Nephrol 2020;21(1):1-6.
[Crossref] [Google Scholar] [Pub Med]
- Louis DW, Kolte D, Kennedy K, Lima FV, Abbott JD, Shemin D, et al. Thirty-day readmission after medical versus endovascular therapy for atherosclerotic renal artery stenosis. Am J Cardiol 2020;125(7):1115-22.
[Crossref] [Google Scholar] [Pub Med]
- Otalora L, Chavez E, Watford D, Tueros L, Correa M, Nair V, et al. Identification of glomerular and podocyte-specific genes and pathways activated by sera of patients with focal segmental glomerulosclerosis. PloS one 2019;14(10):e0222948.
[Crossref] [Google Scholar] [Pub Med]
- Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol 2010;28(5):486-94.
[Crossref] [Google Scholar] [Pub Med]