- *Corresponding Author:
- N. K. Dubey
Centre of Advanced Study in Botany, Institute of Science, Banaras Hindu University, Varanasi-221 005, India
E-mail: nkdubeybhu@gmail.com
| Date of Submission | 30 May 2016 |
| Date of Revision | 29 October 2016 |
| Date of Acceptance | 29 December 2016 |
| Indian J Pharm Sci 2017;79(1): 29-34 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present investigation deals with chemical standardization and aflatoxin inhibitory and antioxidant activity of a herbomineral formulation, sahaj vati prepared in two batches (batch I and II) by mixing shilajeet (thick exudates of the mountain during summer season), guggul (Commiphora mukul (stocks) hook.), haridra (Curcuma longa L) and chitrak (Plumbago zealanica L) with levigation of Agnimantha kwatha (Premna mucronata L). Batch I was prepared by mixing shilajeet, guggul, haridra and chitrak in equal proportion (25% each by weight) while batch II comprised shilajeet (44.7%), guggul (44.7%), haridra (4.8%) and chitrak (5.8%) by weight. Sahaj vati II showed highest (96%) inhibition of aflatoxin production at concentration of 10 000 µg/ml. Suddha guggul exhibited strong antioxidant activity showing lowest IC50 at concentration of 20.56 µg/ml. Based on these findings, the newly formulated herbomineral formulation could be recommended for value addition of nutraceuticals by enhancing their shelf life against lipid per oxidation and aflatoxin contamination.
Keywords
Herbomineral formulation, shahaj vati, antiaflatoxigenic, antioxidant, nutraceuticals
Aflatoxins (AF) are highly health hazardous carcinogenic toxins produced by some species of Aspergillus viz. Aspergillus flavus and A. parasiticus. These are difuran containing polyketides having various physiological effects on both plants and animals. In humans and animals, AF cause intoxication, listlessness, anorexia, vomiting, abdominal pain and haemorrhage, pulmonary oedema, acute liver damage, loss of digestive tract function, convulsions, cerebral oedema, coma and finally death [1]. In addition, AF are also hepatotoxic, carcinogenic and mutagenic in nature [2,3]. Among different AF, aflatoxin B1 (AFB1) is the most potent in terms of its hazardous effects on human health. The aflatoxin secreting strains of A. flavus are prevalent in tropical and subtropical countries and are found to be associated with our different food items which cause serious food poisoning to human being. Moreover, due to unscientific post-harvest processing, there are reports on aflatoxin contamination of different herbal raw materials and nutraceuticals used for preparation of herbal drugs [4]. Congenial environmental conditions in tropical and subtropical countries favour the growth of common aflatoxin producing fungi [5]. Moreover, AF also accelerate oxidative stress resulting in increase of toxic reactive oxygen species and generation of enumerable free radicals leading to lipid peroxidation, thereby, suggesting a positive correlation between oxidant concentration and aflatoxin biosynthesis in food items as well as Ayurvedic preparations [6,7].
Hence, there is need to search some safe curative measures against aflatoxin secretion and oxidative stress so as to enhance the shelf life of Ayurvedic formulations, nutraceuticals and food items. In the present study three plant-based drugs, haridra (Curcuma longa L.), chitrak ( Plumbago zeylanica L.), guggul ( Commiphora mukul (stocks and hook.) and shilajeet (thick exudates of the mountain during summer season) from mineral origin have been selected for testing as antiaflatoxin and antioxidant activity. All the aforementioned drugs have been reported to possess antiinflammatory and antiobesity properties [8,9]. In addition, crude haridra has been reported to be antiallergic [10]; chitrak to be hepatoprotective [11]; guggul could act as a drug delivery system [12] and shilajeet reported to increase oxygen availability to the cells [13]. Hence, the present investigation dealt with the preparation of a standardized formulation coded ‘sahaj vati’ by mixing all the aforementioned drugs in equal proportion applying Ayurvedic technology and evaluation of its antiaflatoxin and antioxidant activity.
Material and Methods
All the ingredients of sahaj vati were procured from local Ayurvedic market (Goladinanath, Varanasi, Uttar Pradesh, India) except guggul, which was procured from Jaipur, Rajsthan. Plant materials were authenticated in the Department of Botany, Banaras Hindu University (BHU), Varanasi, India and voucher specimens were kept in the herbarium of the Laboratory of Herbal Pesticide, Department of Botany, BHU, Varanasi, India. Voucher specimen no. for haridra, chitrak, guggul was zinziber 2014/2, plumbagin 2015/1 and Bursera 2015/1, respectively. Shilajeet sample was authenticated in the Department of Ras Shashtra, Faculty of Ayurveda, Institute of Medical Sciences, BHU, Varanasi, India.
Chloroform, methanol, potato dextrose agar (PDA) medium (potato 200 g; dextrose 20 g; agar 18 g and distilled water 1000 ml), sucrose, magnesium, potassium and yeast (SMKY) medium (sucrose 200 g; MgSO4·7H2O, 0.5 g; KNO3 0.3 g and yeast extract 7 g; 1 l distilled water), toluene:isoamylalcohol:methanol in ratio of 90:32:2; v/v/v (TIM), and 2,2-diphenyl-1- picrylhydrazil (DPPH) were procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Silica gel G for thin layer chromatography (TLC) was procured from SRL, Mumbai, India. Centrifuge, UV transilluminator (Zenith Engineers, Agra, India) and a spectrophotometer (Systronics India Ltd., Mumbai, India) were employed. Aflatoxigenic strain LHP-PV-1 of A. flavus isolated from Pistachia vera has been used for analysis of antiaflatoxigenic activity of drugs.
Preparation of sahaj vati
Before preparing sahaj vati, Samples of haridra and chitrak were dried in an oven at 40° and the powder was prepared by milling process. Shodhan (purification) of shilajeet and guggul was done as per Ayurvedic concepts to get suddha (pure) shilajeet and suddha guggul. Then two batches of sahaj vati were prepared as per standard operative procedure by using suddha shilajeet, suddha guggul, haridra and chitrak as main ingredients and seven Bhavana (levigation) of Agnimantha kwatha (decoction) with slight modification in pharmaceutical technology [14]. Batch I was prepared by mixing shilajeet, guggul, haridra and chitrak in equal proportion (25% each by weight) and then levigated by decoction of Agnimantha for seven times. Bath II was prepared by mixing shilajeet (44.7%), guggul (44.7%), haridra (4.8%) and chitrak (5.8%) by weight and then levigated by decoction of Agnimantha for seven times.
Moisture content and pH
To calculate moisture content, 50 g of each herbal drug sample was dried at 100° in a hot air oven for 24 h and difference with the first weight was calculated [15]. For measuring pH of each preparation, 1 g of each sample was finely ground by a sterilized mixer grinder. A suspension of each sample was prepared in ratio 1:10 (sample:distilled water, w/v), stirred for 24 h and then the pH was measured.
Phytochemical analysis for estimation of alkaloids, tannins, glycosides, terpenes, phenol, flavanoids and sugar was performed using the method reported by Afaq et al.
Alkaloids
To detect the presence of alkaloids, few drops of Mayer’s reagent was added in solvent free extracts. Alkaloid solution produced cream coloured precipitate in presence of Mayer’s reagent. Fifty milligram solvent free extract was stirred with few ml of dilute HCl and filtered. To the filtrate, 1 or 2 ml of Hager’s reagent was added. A prominent yellow precipitate indicated the presence of alkaloids.
Phenols and tannins
One millilitre of the extract and 2 ml of distilled water were taken in a test tube and a few drops of 10% ferric chloride (FeCl3) were added. Appearance of blue or green colour indicated presence of phenols. The extract (50 mg) was dissolved in distilled water and to this 3 ml of 10% lead acetate solution was added. A bulky white precipitate indicated the presence of phenolic compounds. One millilitre of 5% FeCl3 was added to solvent free extract in a test tube. The presence of tannin was indicated by the formation of bluish black or greenish black precipitate.
Sugars
The extract was dissolved in 5 ml of water and filtered. To 0.5 ml of filtrate, 0.5 ml of Benedict’s reagent was added. The mixture was heated on boiling water bath for 2 min. A characteristic coloured precipitate indicated the presence of sugars.
Glycosides and saponins
Five millilitres of the extract was treated with 2 ml of glacial acetic acid containing a drop of FeCl3 solution. Afterwards, it was underplayed with 1 ml concentrated sulphuric acid (H2SO4). A brown ring of the interface indicated a deoxy sugar characteristic of cardenolides. Fifty milligrams of extract was hydrolysed with concentrated HCl for 2 h on a water bath, it was then filtered. In 2 ml of filtrate, 3 ml of chloroform was added and shaken. The chloroform layer was separated and 10% ammonia was added to it. Pink colour indicated the presence of glycosides. The extract was diluted with 20 ml distilled water and was agitated in a graduated cylinder for 15 min. The formation of 1 cm layer of foam indicated the presence of saponins.
Antiaflatoxigenic activity of sahaj vati and its ingredients
Requisite amounts of sahaj vati and its individual ingredients were added to 25 ml SMKY to achieve various concentrations ranging from 1000 to 10 000 μg/ml. The medium was inoculated separately with 20 μl spore suspension (106 spores/ml) of toxigenic strain, LHP-PV-1 of A. flavus isolated from P. vera and was incubated for ten days at 27±2°. The medium was filtered (Whatman No. 1) and the mycelia was dried at 80° for 12 h for calculating dry weight. Thereafter, the content of each flask was filtered and the filtrate was extracted with 20 ml chloroform. The extract was then evaporated to dryness on a water bath at 60° and re-dissolved in 1 ml chloroform. AFB1 was detected by TLC. For this, 50 μl of chloroform extract was loaded on TLC plates along with the standard of AFB1 and developed in toluene:isoamylalcohol:methanol (90:32:2; v/v/v). The plate was air dried and AFB1 spots were observed in UV transilluminator (360 nm). The amount of aflatoxin secreted by the A. flavus LHPPV- 1 in the medium was quantified by TLC followed by spectrophotometry. The blue spots on TLC plates were scrapped, dissolved in 5 ml of methanol and centrifuged at 5000 rpm for 5 min. Absorbance of the supernatant was recorded at 360 nm and AFB1 was calculated following the method reported by Kumar et al. [16] AFB1 (μg/l) = (D×M)/(E×L)×1000, where D was the absorbance, M the molecular weight (312), E was the molar extinction coefficient of AFB1 (21800) and L the path length (1 cm).
Free radical scavenging activity
Free radical scavenging activity of sahaj vati and its individual ingredients were measured by recording the extent of bleaching of the purple-coloured DPPH solution to yellow following the method reported by Prakash et al. [17]. Different concentrations (10.00-400 μg/ml) of sahaj vati and its ingredients were added to 5 ml of 0.004% DPPH solution in methanol. After 30 min incubation at room temperature, the absorbance was measured against a blank at 517 nm using a spectrophotometer. Scavenging of DPPH free radical with reduction in absorbance of the sample was taken as a measure of their antioxidant activity IC50, which represented the concentration of sahaj vati and its ingredients that caused 50% neutralization of DPPH radicals was calculated from the graph plotting between percent inhibition and concentration. I% = (Ablank– Asample/Ablank)×100, where, Ablank is the absorbance of the control (without test compound), and Asample is the absorbance of the test compound.
Statistical analysis
Experiments were performed in triplicate and data analysed are mean±SE subjected to one way ANOVA. Means are separated by the Tukey's multiple range tests when ANOVA was significant (P<0.05) with the help of SPSS 16.0; IBM Corporation.
Results and Discussion
Moisture content and pH of sahaj vati and its ingredients, pH of all the samples were found to be in the acidic range where suddha shilajeet was least acidic (pH=6.67) and suddha guggul was strongly acidic (pH=3.77). Moisture content was maximum in chitrak powder (6.46%) and suddha guggul along with batch II of sahaj vati possessed least moisture (0.24%) (Table 1).
| Sample Name | pH | Moisture content |
|---|---|---|
| Suddha shilajeet | 6.67 | 0.24 |
| Suddha guggul | 3.37 | 0.90 |
| haridra | 5.68 | 6.21 |
| chitrak | 4.50 | 6.46 |
| sahaj vati batch I | 4.87 | 2.93 |
| sahaj vati batch II | 5.03 | 0.24 |
Table 1: Moisture Content and PH of Sahaj Vati and Its Ingredients
Qualitative phytochemical analysis of both batches of sahaj vati and their ingredients revealed the presence of polyphenols, saponins, terpenes and steroids. However, batch I of sahaj vati also contained alkaloids. Saponins and alkaloids were found in haridra and chitrak while in suddh shilajeet only steroids and terpenes were present. Steroids, terpenes, phenol and saponins were found in suddha guggul (Table 2).
| Name of Samples | Alkaloids | Steroids | Terpene | Sugar | Phenol | Saponins |
|---|---|---|---|---|---|---|
| Suddha shilajeet | Absent | Present | Present | Absent | Absent | Absent |
| Suddha guggul | Absent | Present | Present | Absent | Present | Present |
| haridra | Present | Absent | Absent | Absent | Absent | Present |
| chitrak | Present | Absent | Absent | Absent | Absent | Present |
| sahaj vati batch I | Present | Present | Present | Absent | Present | Present |
| sahaj vati batch II | Absent | Present | Present | Absent | Present | Present |
Table 2: Qualitative Phytochemical Analysis of Sahaj Vati and Its Ingredients
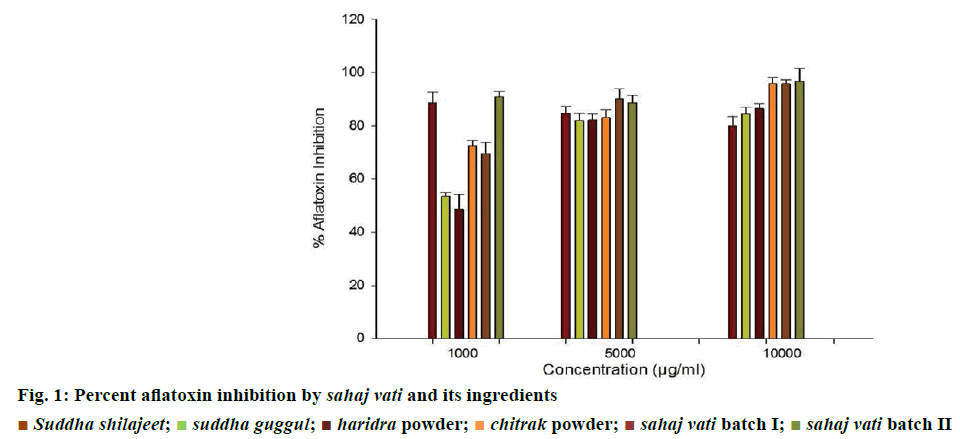
The results obtained on antiaflatoxigenic effects of the tested sahaj vati and its plant-based ingredients revealed that among 21 samples, sahaj vati II showed maximal inhibition of aflatoxin production (96%) at a concentration of 10 000 μg/ml and haridra powder exhibited lowest inhibition of aflatoxin production (48%) at a concentration 1000 μg/ml (Figure 1).
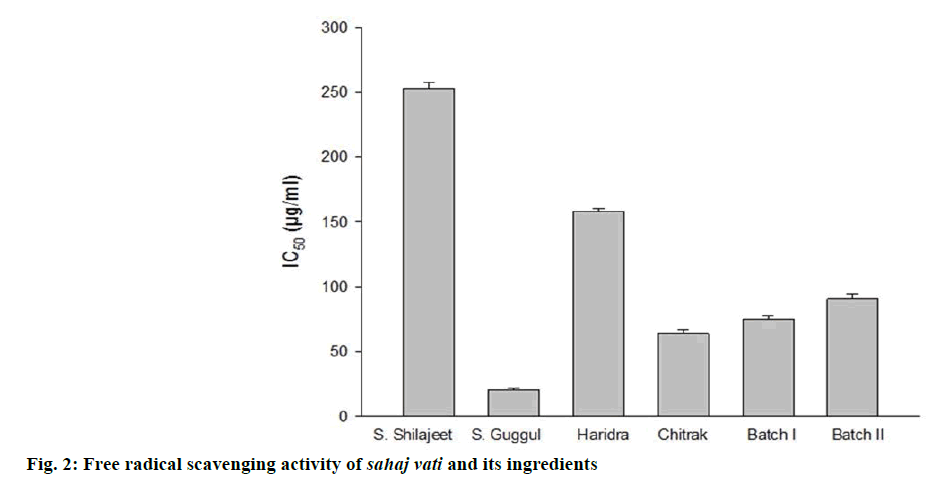
The discoloration of the purple colour of the DPPH confirmed the positive antioxidant activity of sahaj vati and its ingredients. All of these showed free radical scavenging in a dose-dependent manner. Free radical inhibitory activity and IC50 values have been summarized in Figure 2. Sudha guggul exhibited strong antioxidant activity showing lowest IC50 at concentration of 20.56 μg/ml while suddha shilajeet showed poor antioxidant activity having highest IC50 at concentration of 253.12 μg/ml.
Ayurveda system of medicine is recognized as one of the oldest medical systems of the world [18] and its medicines are mainly derived from plants, metals and minerals as well as from animals. Ancient texts of Ayurveda such as Atharvaveda, Charak Samhita and Sushrut Samhita together described more than 700 medicinal herbs, categorized as per their taste, appearance, digestive effects, safety, efficacy, dosage and benefits [19]. Ayurvedic drugs are gaining much popularity for a number of reasons that these drugs are expected not to possess unacceptable side effects of modern drugs and not to facilitate development of drug-resistant microorganisms [20]. Most of the Ayurvedic preparations are of herbal origin, which are multi component in nature and prevent development of resistance in pathogens. Hence, for countering aflatoxin secretions in herbal formulations and nutraceuticals, these herbal drugs would be a better option because of the synergistic nature of their components and multiple modes of action. Based on the findings of the present study, sahaj vati II, which showed highest (96%) inhibition of aflatoxin production could be recommended for formulation of drugs to reduce food poisoning by aflatoxicoses. Moreover, Suddha guggul, which exhibited strong free radical scavenging activity as evidenced by the lowest IC50 might be recommended for use as an antioxidant for enhancing shelf life of herbal formulations and nutraceuticals.
Although, the dose of the crude formulation of sahaj vati was comparatively high, the formulation had some merits over antibiotics, which are however effective in low doses. In case of antibiotics there have been frequent reports of emergence of resistance in target microbes as a result, these antibiotics might go out of use after a period of time. Sahaj vati might not face that problem for reasons that have already been documented in this discussion.
Moreover, in the herbal system, no reports could be found in the literature on the use of plant-based formulations to counter the problem of aflatoxin contamination. The formulations developed in the present investigation require further evaluation and if necessary even clinical trials to establish their utility beyond reasonable doubt. These formulations contain mixtures of different plant samples, which are expected to act synergistically with least chances of resistant strains emerging from the food borne fungi. The formulation might be also used in post-harvest processing of herbal raw materials and nutraceuticals to check their fungal and aflatoxin contamination. Hence, the formulations may be recommended to agri firms as well as pharmaceutical firms to have a wider market. The herbal products used in the formulation are easily availability as they grow widely in India hence rendering the formulations economical to produce. Further research is also required to test seasonal variations in aflatoxin inhibiting efficacy of the drug samples as most of Ayurvedic medicines exhibit seasonal variations. The antioxidant activity of the drug sample suddha guggul allows its application as an ingredient in different herbal formulations to check their deterioration due to lipid peroxidation. The drug samples were chemically standardized in the present investigation, which is desirable before clinical trial of a newly developed formulation. The antioxidant activity of the formulation might be due to the presence of polyphenolics. The formulations have also been standardized by determining their pH, which was in the acidic range as well as by determining their moisture contents. These protocols must be established for raw material standardization, which would help in the preparation of standardized formulation with desirable efficacy for clinical trials and human application. Further clinical and pharmacological trials are required with the newly prepared formulation. The traditional use of individual drug samples for a long time strengthens a possibility of favourable safety profile of the formulation.
Based on the results of the present investigations, the herbomineral formulation, Sahaj vati and Suddha guggul has the potential to be recommended as novel plant-based formulations against aflatoxicosis and oxidative stress, respectively for addition to nutraceuticals to enhance shelf life. Needless to state that further extensive preclinical and clinical evaluations are necessary before these recommendations could be implemented.
Acknowledgements
Authors thank Prof. N. K. Dubey, Department of Botany, BHU for authenticating the samples of haridra, chitrak, and guggul and Prof. A. K. Chaudhary, Department of Ras Shashtra, Faculty of Ayurveda, BHU, for authenticating the sample of shilajeet. Authors also thank the head, Centre of Advanced Study in Botany, Institute of Science, and head, Department of Rasa Shastra, Faculty of Ayurveda, Institute of Medical Sciences, BHU, Varanasi for providing laboratory facilities and the CSIR, New Delhi for the financial assistance.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Gong Y, Hounsa A, Egal S. Post weaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ Health Perspect 2004;112:1334-8.
- Basmacioglu G, Oguz H, Ergul M, Col R, Birdane YO. Effect of dietary esterified glucomannan on performance, serum biochemistry and haematology in broiler exposed to aflatoxin. Czech J Anim Sci 2005;20:31-9.
- Wagacha JM, Muthomi JW. Mycotoxin problem in Africa current status implications to food safety and health and possible management strategies. Int J Food Microbiol 208:124:1-12.
- Mishra PK, Kedia A, Dubey NK. Chemically characterized Cymbopogon martinii (Roxb.) Wats. essential oil for shelf life enhancer of herbal raw materials based on antifungal, antiaflatoxigenic, antioxidant activity and favourable safety profile. Plant Biosystems 2015;150:1313-22.
- Dubey NK, Srivastava B, Kumar A. Current Status of Plant Products as botanical pesticides in storage pest management. J Biopesticides 2008;1:182-6.
- Reverberi M, Zjalic S, Racell A, Fabbri AA, Fanelli C. Oxidant/antioxidant balance in Aspergillus parasiticus affects aflatoxin biosynthesis. Mycotoxin Res 2006;22:39-47.
- Dwivedy AK, Kedia A, Kumar M, Dubey NK. Essential oils of traditionally used aromatic plants as green shelf life enhancers for herbal raw materials from microbial contamination and oxidative deterioration. Cur Sci India 2016;110:143-5.
- Shehzad A, Ha T, Subhan F, Lee YS. New mechanisms and the antiinflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 2011;50:151161.
- Kotiyal JP, Singh DS, Bisht DB. Gum Guggul (Commiphora mukul) fraction A in obesity-a double blind clinical trial. J Res Ayur Siddha 1985;6:20-35.
- Kurup VP, Barrios CS. Immunomodulatory effects of curcumin in allergy. Mol Nutr Food Res 2008;52:1031-9.
- Gupta A, Gupta A, Singh J. Note New Naphthoquinones from Plumbago zeylanica. Pharm Biol 1999;37:321-3.
- Yadav KD, Chaudhary AK. New world syndrome (obesity) gone by guggul: a review. J Ayurveda Holistic Med 2014;2:31-5.
- Yadav KD, Chaudhary AK. Shilajeet for obesity: A probable pharmacological postulate. Int J Res Ayurveda Pharm 2015a;6:69-72.
- Yadav KD, Chaudhary AK. Pharmaceutical Postulates for Standard Operative Procedures to prepare Sahaj Vati. Int J Pharm Biol Sci Arch 2015b;6:37-41.
- Mandeel QA. Fungal contamination of some imported spices. Mycopathologia 2005;159:291-8.
- Kumar R, Mishra AK, Dubey NK, Tripathi, YB. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int J Food Microbiol 2007;115:159-64.
- Prakash B, Shukla R, Singh P, Kumar A, Mishra PK, Dubey NK. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int J Food Microbiol 2010;142:114-9.
- https://nccih.nih.gov/health/ayurveda/introduction.htm.
- Padma TV. Ayurveda. Nature 2005;436:486-6.
- Patwardhan B, Vaidya ADB, Mukund C. Ayurveda and natural products drug discovery. Curr Sci India 2004;86:789-99.
 Suddha shilajeet;
Suddha shilajeet;  suddha guggul;
suddha guggul;  haridra powder;
haridra powder;  chitrak powder;
chitrak powder;  sahaj vati batch I;
sahaj vati batch I;  sahaj vati batch II
sahaj vati batch II