- Corresponding Author:
- A. Chaitanya krishna
Department of Bioanalaysis, Bombay Bioresearch Center, Govandi, Mumbai-400 043, India
E-mail: chaitanya@bbrc-cro.com
| Date of Submission | 16 February 2012 |
| Date of Revision | 14 December 2012 |
| Date of Acceptance | 18 December 2012 |
| Indian J Pharm Sci., 2012, 74 (6): 541-548 |
Abstract
A simple, rapid, specific and sensitive liquid chromatography tandem mass spectrometric method has been developed and validated for the determination of doxycycline from the human plasma. Doxycycline is extracted from human plasma by solid phase extraction. Demeclocycline was used as an internal standard. Detection was performed at transitions of 444.800→428.200 for doxycycline and 464.700→448.100 for demeclocycline using mass spectrometry. Chromatographic separation of analyte and internal standard were carried out using a reverse phase C18, column at 0.500 ml/min flow. The assay of doxycycline is linear over the range of 0.055-7.612 μg/ml, with a precision <14.83%, regression coefficient (r 2 ) ]=0.9961 and the limit of quantification in plasma for doxycycline was 0.055 μg/ml. Mean extraction recovery obtained was 95.55%. Samples are stable at room temperature for 6 h, processed samples were stable at least for 30.20 h and also stable at three freeze-thaw cycles. The method has been used to perform pharmacokinetic and bioequivalence studies in human plasma.
Keywords
Doxycycline, human plasma, LC-MS/MS, solid phase extraction, validation
Doxycycline is a semisynthetic tetracycline broadspectrum antibiotic frequently used to treat chronic prostatitis, syphilis, sinusitis, chlamydia, pelvic inflammatory diseases, rickettsial infections, sexually transmitted diseases. Doxycycline chemically is [4S-(4α,4aα,5α,5aα,6α,12aα)]-4- (dimethylamino)- 1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a? pentahydroxy-6-methyl-1,11-dioxo-2-naphthalene carboxamide monohydrate [1]. Doxycycline is a yellow crystalline powder with a molecular weight of 444.45 g/mol. Its empirical formula is C22H24N2O8 and is sparingly soluble in water, slightly soluble in alcohol and in methylene chloride.
Very few HPLC [2-4], LC-MS methods [5] have been applied for the determination of doxycycline in plasma, and in other biological matters in different methods [6-12], but no method has been found and performed for doxycycline by LC-MS/MS in human plasma. Hence, the authors have proposed, developed and validated a simple, rapid, specific, sensitive LC-MS/MS method to determine doxycycline in human plasma.
Materials and Methods
Doxycycline is obtained from Svizera Labs Pvt. Ltd., Mumbai. The internal standard demeclocycline is purchased from Clearsynth Labs Pvt. Ltd., Mumbai. Methanol (HPLC grade), acetonitrile (HPLC grade), potassium dihydrogen phosphate (GR grade), trifluoroacetic acid (AR grade), water (Ultra Pure grade), PLEXUS 30 mg/1cc cartridges (Analchem) were used.
The liquid chromatography coupled with tandem mass spectrometer (LC-MS/MS) system consists of a Finnigan surveyor autosampler, surveyor LC pump plus solvent delivery system and a column oven (Thermo Electron Corporation) used for ion separations. The mass spectrometer was thermo scientific TSQ quantum discovery max ultra triple stage quadrupole mass spectrometer used for ion detection. An electron spray ionization (ESI) source was used. Data was collected and processed using LC Quan Version. 2.5.6 Data collection and integration software.
Chromatographic condition
The liquid chromatographic separations were carried out by using Cadenza CD-C18 (30×4.6 mm), 3μ column (Imtakt). Column temperature was held at 30°. The auto sampler tray temperature was 10°. The mobile phase is composed of 0.04% TFA in (methanol:acetonitrile:water (47.5:47.5:5)) v/v with flow rate of 0.500 ml/min. A typical injection volume of 2 μl is injected into the mass spectrometer for detection.
MS/MS detection
Precursor ions for analyte and internal standard were determined from mass spectra obtained by the TSQ mass spectrometer, which includes an electronically-controlled, integrated syringe pump. The MS conditions for doxycycline and the internal standard were optimized by separate infusion into the MS at a flow rate of 5 μl/min constantly while adjusting MS parameters to achieve maximal intensity. Electro-spray ionization in positive ion mode (ESI+) was used for ionization and selective reaction monitoring (SRM) mode was chosen for detection. The optimized precursor ions pairs were m/z 444.800→428.200 for doxycycline and m/z 464.700→448.100 for demeclocycline. The optimized MS parameters were as follows:ion spray voltage: 5000 V, sheath gas pressure: 45 psi, auxiliary gas pressure: 15 psi, capillary temperature: 350°. collision pressure: 0.8 psi. Peak areas were automatically integrated using LC Quan version 2.5.6 (Thermo Corporation).
Preparation of calibration standards and quality control samples
The calibration standards and the quality control (QC) samples were prepared from separate stock standard solutions. The concentrations of stock solutions were 1.001 mg/ml and 0.988 mg/ml for calibration standard and quality control samples, respectively and methanol was used as diluent. The spiking solutions for calibration standards and quality control concentrations were prepared in methanol:water (1:1). The calibration standard human plasma samples were prepared by spiking corresponding spiking calibration standard solutions into blank human plasma to provide concentrations range between 0.055 to 7.612 μg/ml. For quality control plasma samples preparation, the corresponding solutions were spiked to the human blank plasma to attain the concentration of 0.056, 0.161, 3.369 and 5.434 μg/ml for limit of quantification quality control samples (LOQQC), low quality control samples (LQC), middle quality control samples (MQC), high quality control samples (HQC), respectively. For preparation of the samples, volumes of 20 μl were spiked into 980 μl of human blank plasma. Internal standard stock solution (935.412 μg/ml) of demeclocycline was prepared in methanol. Working internal standard solution (30.000 μg/ml) was prepared by diluting in methanol:water (1:1).
Sample extraction
A 150.0 μl aliquot of plasma samples was mixed with 50.0 μl of internal standard working solution (30.0 μg/ml) and pretreatment is performed by adding 0.6 ml of potassium dihydrogen phosphate and vortexed to mix the samples for approximately 2 min and adopted the following SPE procedure. A commercially available cartridge (Analchem PLEXUS 30 mg/1cc cartridges) was utilized for extraction. After conditioning and equilibrating the cartridge with 1 ml of methanol and water, respectively, the drugs were extracted into the cartridge by loading the pre-treated plasma samples. Then the cartridges were washed using 2×1.0 ml of 5% methanol, in order to wash the unbound substance in the cartridge and reduce any interfering band in chromatograms. Finally the drug is eluted from the cartridge with 0.5 ml of mobile phase then subjected 2.0 μl samples for chromatographic analysis.
Validation
Blank human plasma from eight different lots (including one hemolyzed and one lipemic) [13] were processed without analyte and internal standard. And with the same eight lots, LLOQ level is processed to evaluate the presence of any interference at the retention time of analyte and internal standard.
Matrix factor
Evaluation of the matrix factor at low and high quality control concentrations were done to ensure that the precision, selectivity and sensitivity are not compromised due to a change in matrix. Matrix factor can be termed as the quantitative measurement of the matrix effect. Aqueous mixtures of internal standard and analyte at concentrations representing 100% extraction of internal standard and analyte at LQC and HQC concentrations were prepared. These shall serve as reference samples. Processed duplicate eight different lots of blank matrices (from eight individuals, including, one hemolyzed and one lipemic) [13] without addition of internal standard. Eluted solution volumes were equally diluted with reference sample; it is compared with respective aqueous reference sample equally diluted with mobile phase.
Calibration curve and linearity
The eight point calibration curve was constructed by plotting, peak area ratio of doxycycline to their corresponding internal standard versus doxycycline concentrations. A linear regression with weighing factor of linear 1/x2 was applied.
Intra- and inter-day assay accuracy and precision
Intra-day precision and accuracy were determined by analysis of six replicates of each QC sample (n=6) at LOQQC, LQC, MQC and HQC concentration levels extracted with a set of standards in one batch. The same procedure was repeated on different day with new samples to determine inter-day precision and accuracy.
Recovery
Recovery is carried out to evaluate the loss of drugs and/or internal standards during sample extraction. The drugs and internal standards area counts from extracted QC samples were compared with corresponding QCs reference samples to evaluate any loss of either drugs or internal standards. It is preferable to observe consistent recovery for all three QC levels.
Stability
Stability of both drugs in different matrices and under different conditions was evaluated. The detailed tests are described below. Stability was assessed by comparing the mean concentration of the stored QC samples with the mean concentration of freshly prepared QC samples. Drug stability in pooled human blank plasma is a function of the storage conditions, the chemical properties of the drug and the matrix effect. The following tests were performed to evaluate the stability, short term and long term stock solution stability, bench top stability, freeze and thaw stability, autosampler stability, wet extract stability, long term stability in matrix.
Results and Discussion
The results of the method validation are summarized from Table 1 and 4. Table 1 represents results of the calibration standards and linearity. For three consecutive batches, the calibration curves showed an overall accuracy of 96.04-106.48% with relative standard deviation (RSD) of 0.77-8.94%. The calibration standard linearity has a regression coefficient of >0.9961.
| Batch ID | Theoretical concentration (µg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| 55.500 | 111.100 | 555.400 | 1461.500 | 2923.000 | 4871.700 | 6089.700 | 7612.100 | |
| Batch 01 | 52.143 | 127.054 | 482.498 | 1531.122 | 2958.406 | 4778.324 | 6030.918 | 7743.066 |
| Batch 02 | 53.084 | 121.305 | 547.461 | 1451.494 | 2822.347 | 4741.425 | 6247.425 | 7673.729 |
| Batch 03 | 56.535 | 106.546 | 570.290 | 1409.701 | 3018.640 | 4628.825 | 6372.171 | 7625.189 |
| Mean | 53.921 | 118.302 | 533.416 | 1464.106 | 2933.131 | 4716.191 | 6216.838 | 7680.661 |
| Precision (%) | 4.29 | 8.94 | 8.54 | 4.21 | 3.43 | 1.65 | 2.78 | 0.77 |
| Accuracy (%) | 97.15 | 106.48 | 96.04 | 100.18 | 100.35 | 96.81 | 102.09 | 100.90 |
Table 1: Accuracy and Precision for Calibration Standards
There was no significant interference observed during the retention time of analyte and internal standard while performing selectivity and specificity.
The method was found to be highly accurate and precise. For doxycycline, accuracy of 87.8-115.3% and precision of 2.4-16.8 % for intra-assay, and accuracy of 91.1-106.5% and precision of 4.4-14.8% RSD for inter-assay were obtained for all QC levels including LOQQC. The results of inter and intraday accuracy and precision are summarized in Table 2.
| Parameter (%) | Intra-batch | Inter-batch |
|---|---|---|
| Accuracy | 87.8-115.3 | 91.1-106.5 |
| Precision | 2.4-16.8 | 4.4-14.8 |
Table 2: Intra and Inter-Day Accuracy and Precision
Observed RSD of matrix factor is 7.75% and 5.95% in LQC, 4.90% and 4.79% in HQC for doxycycline and internal standard, respectively. All eight matrix lots showed very similar matrix effect for both analyte and their corresponding internal standard.
An overall recovery of 95.54% for doxycycline, and 99.54% for demeclocycline were obtained. Both compounds show consistent recovery results for all three QC levels. Results are summarized in Table 3.
| QC Level | Recovery (%) | |
|---|---|---|
| Doxycycline | Demeclocycline | |
| LQC | 91.52 | 96.42 |
| MQC | 98.63 | 100.79 |
| HQC | 96.49 | 101.42 |
| Recovery | 95.55 | 99.54 |
| %CV | 3.82 | 2.74 |
Table 3: Recovery of Analyte and Is
Stability of doxycycline in human plasma under different conditions was evaluated. The detailed results are shown in Table 4. As seen from the table, three freeze/thaw cycles, 6 h room temperature storage, 83 days storage at -70° and 28 h auto sampler stability has been established. In addition, 15 days stability for stock standard solutions and wet extract stability for 30.20 h were established. All of these demonstrate the ruggedness of the method.
| Parameter | Bench top stability | Auto sampler stability | Wet extract stability | Freeze and thaw stability | Long term stability | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| QC levels | LQC | HQC | LQC | HQC | LQC | HQC | LQC | HQC | LQC | HQC |
| Precision | 4.32 | 3.51 | 5.34 | 2.14 | 7.75 | 4.71 | 8.67 | 3.08 | 6.02 | 7.00 |
| % Stability | 102.25 | 104.68 | 106.73 | 109.47 | 104.01 | 105.88 | 109.02 | 104.12 | 93.56 | 93.13 |
Table 4: Stability of Doxycycline
During the method development the compounds to be quantified needs a better optimization starting from the selection of detector, mobile phase, column, extraction procedure and other chromatographic conditions.
Optimization of the detectors response is always detrimental factor in the quantification of analyte in a specific media. As the mass detectors are supposed to detect the monoisotopic mass of the charged species of the analyte, it is always necessary to tune the compound for proper identification of parent and daughter mass.
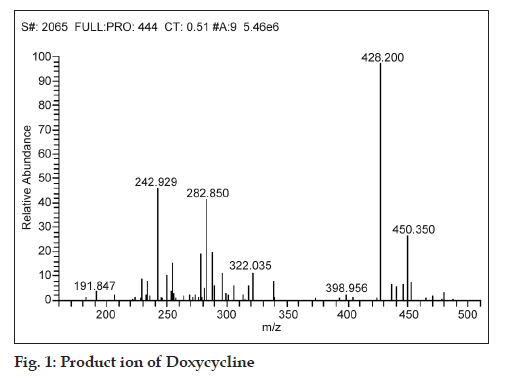
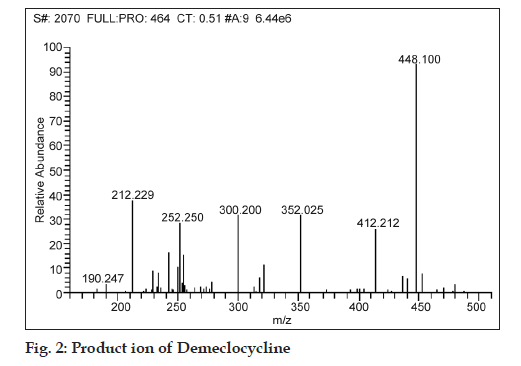
While fragmenting in MS/MS scan mode, the product ions of m/z 242.929, 282.850, 322.035 and 428.2 were observed for a parent ion of doxycycline (m/z 444.800), where in the spectra of m/z 428.2 was found to be more consistent and predominant (fig. 1). Simultaneously, a product ion of m/z 448.1 was found to be more reproducible and intense for demeclocycline precursor ion (m/z 464.7) (fig. 2).
Literature sources have shown that any regular mobile phase will be sufficient for analysis [5,7]. But in practicality, it was learnt that doxycycline was not eluted completely out of the column with different proportions of the mobile phase as specified in the literatures. The base line noise was high and there was no peak observed in the chromatogram window. After trying with all the compositions, it was assumed that different organic modifiers are required apart from regular acidic and basic buffers used on LC-MS/MS. To clarify a HPLC system coupled with UV detector had been used to trouble shoot the problem. Finally, by using both formic acid and trifluoroacetic acid proper improvement in the chromatography has been observed. As trifluoroacetic acid is not a specified organic modifier that can be used in LC-MS/MS, very less amounts (0.04%) had been used to improve the peak shape. With this mobile phase was optimized.
The selection of the internal standard should be decided based on the physicochemical properties of the analyte. In most of the cases, the structural analogue of the analyte will be selected as an internal standard. Though there was a minor difference between the retention time of demeclocycline and doxycycline, matrix effect or ion suppression was not observed.
Trifluoroacetic acid being highly acidic normal columns cannot withstand that particular pH of the mobile phase. It was found that by using both formic acid and trifluoroacetic acid pH was around 1.9. As expected, normal reverse phase columns were unavailable to give proper reproducibility. Trifluoroacetic acid was causing ion suppression, which was undesirable. Even doxycycline was eluting in the void volume. Finally, problems like ion suppression, improper reproducibility, early elution has been eliminated by using Cadenza CD-C18 (30×4.6 mm), 3 μ column (Imtakt).
As a regular practice, protein precipitation has been employed. It was observed that there is matrix effect, ion suppression as well as very low extraction recovery. Due to the difference in the pKa of both drug and internal standard the plasma sample is pretreated with different acidic and basic conditions. It was observed that even protein precipitation or liquid-liquid extraction does not work. Even both liquid-liquid extraction and protein precipitation were used in combination which was not giving a desired result. Different makes of solid phase extraction cartridges (Oasis, Phenomenex, Orochem, Analchem) were used that employ different sorbent chemistries (both reverse phase cartridges and ionic i.e., weak anionic, strong anionic, weak cationic and strong cationic). Finally, it was observed that the matrix effect, recovery problems were being eliminated by using Analchem Plexus cartridges.
In addition to this several LC?MS methods have been reported for doxycycline quantification [5,7,9], where in chromatographic separation was carried out in long columns (100×4.6 mm, 5 μ) resulting in high retention times, thus long run times. As the short column has provided the desirable result during the optimization, the added advantage of low runtime was found to be credible apart from common benefits.
Also detection was held in a single quadrupole mass spectrometer in some of the literatures [5]. Which is less sensitive compared to the detection by using tandem mass spectrometry.
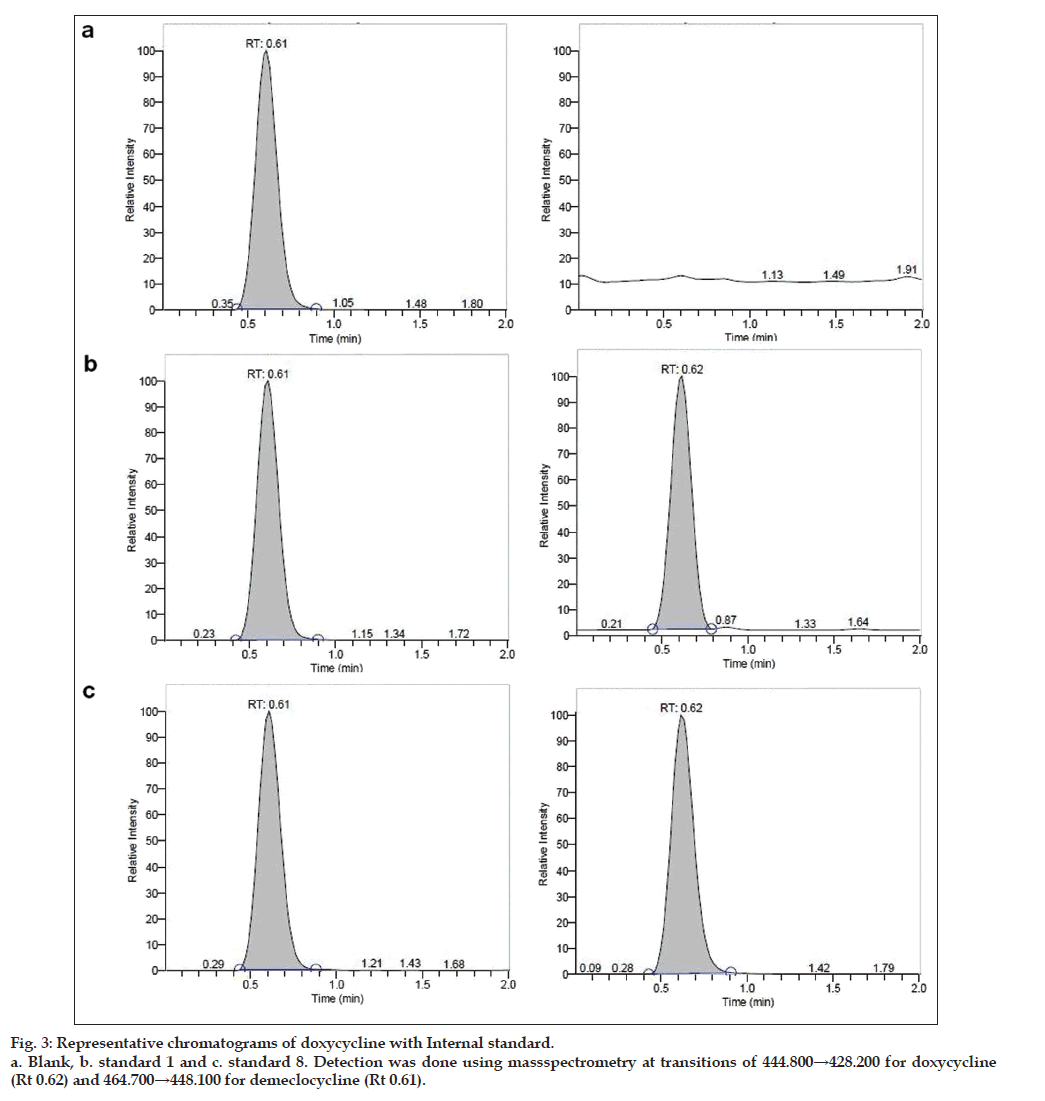
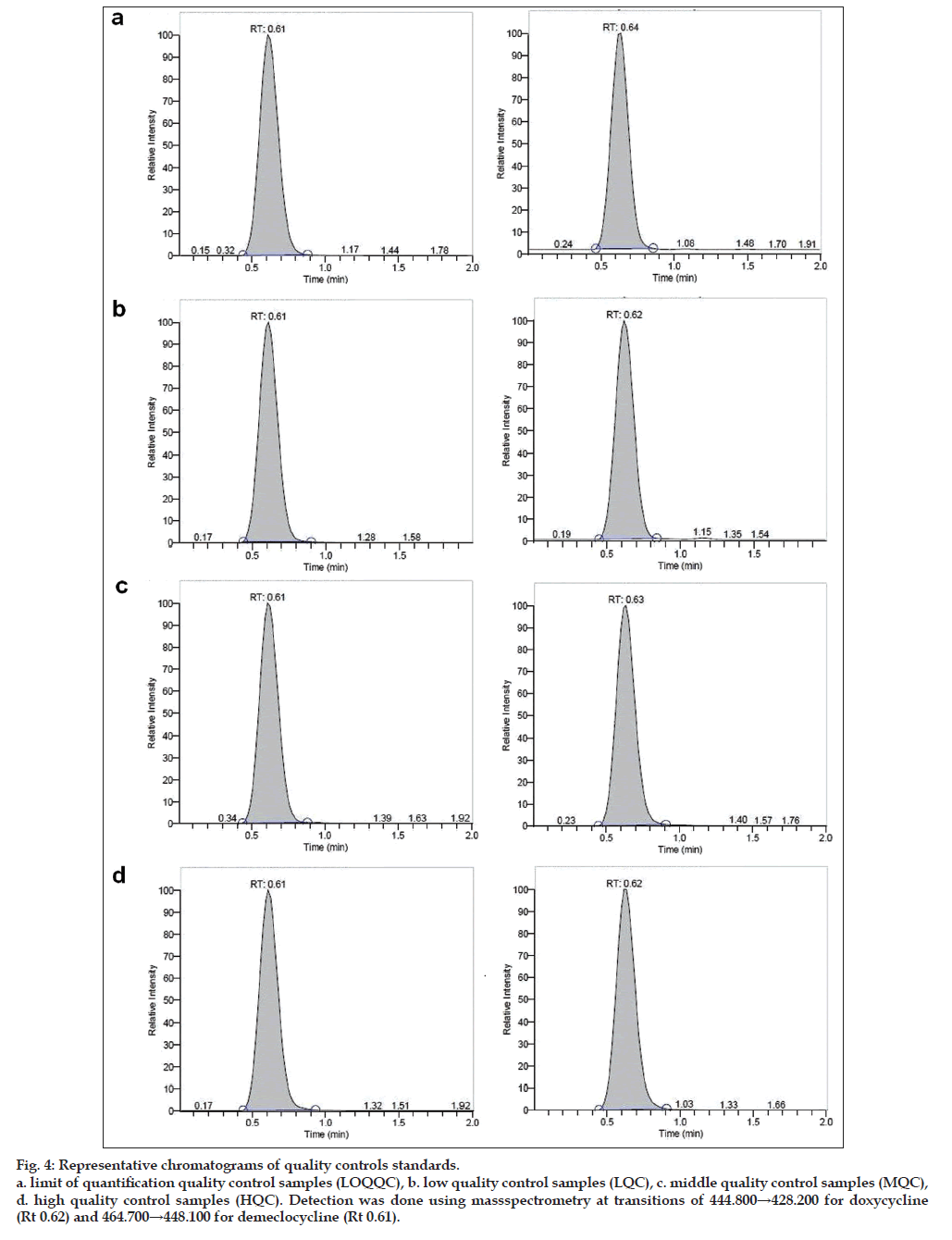
Owing to these disadvantages, authors propose a method developed with shorter run time for analysis. To reduce the run time, small column (30×4.6 mm) 3 μ was used and it was found that both drug and internal standard were resolved fine with a sharp peak shape (figs. 3 and 4).
Fig. 4: Representative chromatograms of quality controls standards.
a. limit of quantification quality control samples (LOQQC), b. low quality control samples (LQC), c. middle quality control samples (MQC),
d. high quality control samples (HQC). Detection was done using massspectrometry at transitions of 444.800→428.200 for doxycycline
(Rt 0.62) and 464.700→448.100 for demeclocycline (Rt 0.61).
Extraction process is carried out by solid phase extraction (SPE) which is a powerful technique for rapid, selective sample preparation. The versatility of SPE allows it to be used for a variety of purposes, such as: purification, trace enrichment, solvent, desalting, derivatization, class fractionation.
This extraction process offers benefits and advantages over other sample preparation techniques such as liquid-liquid extraction [5], including, high and reproducible recovery, highly purified extracts, ease of automation, compatibility with instrumental analysis, productivity enhancement, reduction of organic solvent consumption and extraction time.
The total run time to analyze a sample is 2.0 min, even can be analyzed with 1.5 min as run time as the retention time is 0.61 min for analyte and 0.62 min for internal standard. A simple chromatographic conditions and extraction techniques were adopted which has resulted in a simple, fast and rugged method for the determination for doxycycline to support BA-BE study in human plasma by LC-MS/ MS.
With all these final changes that were made to optimize mobile phase, column and extraction procedures, the major pitfalls of the development like ion suppression and matrix effect, peak shape irregularities etc., were eliminated. Recovery, sensitivity levels, precision and accuracy and chromatograms are found to be with in the acceptance limits.
The developed method is suitable for an accurate determination of doxycycline in human plasma by LC-MS/MS. The effectiveness of the method was proved by the validation data. A simple chromatographic condition and extraction techniques were adapted and results in a simple, fast and rugged method for the determination for doxycycline in human plasma to support BA-BE study for doxycycline. In respect to unavailability of LC-MS/ MS methods for the determination of doxycycline in plasma, this current method is advantageous over reporting methods due to its simplicity in methodology for bioanalysis on LC-MS/MS.
Acknowledgements
We are thankful to Mrs. Gauri Sapte, Director, BBRC and the management of Maneesh Pharmaceuticals Ltd., for their valuable support and contribution in carrying out this research work with out which it will not be possible to publish the paper.
References
- The merck index, 13th edn, Merck Research Laboratories Publication: New Jersey; 2001, p. 606-7.
- Santos MD, Vermeersch H, Remon JP, Schelkens M, De Backer P. Validation of a high-performance liquid chromatographic method for the determination of doxycycline in turkey plasma. J Chromatogr B Biomed Appl 1996;682:301-8.
- Bocker R. Analysis and quantitation of a metabolite of doxycycline in mice, rats, and humans by high-performance liquid chromatography J Chromatogr 1983;274:255-62.
- Ruz N, Zabala M, Kramer MG, Campanero MA, Dios-Viéitez MC. Rapid and simple determination of doxycycline in serum by high-performance liquid chromatography Application to particulate drug delivery systems. J Chromatogr A 2004;1031:295-301.
- Selvadurai M, Meyyanathan SN, Rajan S, Padmanaban G, Suresh B. Determination of doxycycline in human plasma by liquid chromatography-mass spectrometry after liquid-liquid extraction and its application in human pharmacokinetics studies. J Bioequiv Availab 2010;2:93-7.
- Sanderson H, Ingerslev F, Brain RA, Halling-Sørensen B, Bestari J. Dissipation of oxytetracycline, chlortetracycline, tetracycline and doxycycline using HPLC?UV and LC/MS/MS under aquatic semi-field microcosm conditions. Chemosphere 2005;60:619-29.
- Cristina B, Antonio DC, Yolanda P. Determination of tetracyclines in multi-specie animal tissues by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. Food Chem 2009;116:1005-12.
- Cherlet M, De Backer P, Croubels S. Control of the keto-enol tautomerism of chlortetracycline for its straight forward quantitation in pig tissues by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A 2006;1133:135-41.
- Axisa B, Naylor AR, Bell PR, Thompson MM. Simple and reliablemethod of doxycycline determination in human plasma and biologicaltissues. J Chromatogr B Biomed Sci Appl 2000;744:359-65.
- Bogialli S, Coradazzi C, Di Corcia A, Lagana A, Sergi M. A rapid method based on hot water extraction and liquid chromatography?tandem mass spectrometry for analysing tetracycline antibiotic residues in cheese. J AOAC Int 2007;90:864-71.
- Cinquina AL, Longo F, Anastasi G, Giannetti L, Cozzani R. Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle J Chromatogr A 2003;987:227-33.
- Sheridan ME, Clarke GS. Improved high-performance liquid chromatographic determination of doxycycline in serum and urine using solidphase extraction columns. J Chromatogr 1988;434:253-8.
- Guidelines for validation of analytical and Bioanalytical Methods, Brazil, 2003.