- *Corresponding Author:
- Vineeta Khanvilkar
Department of Quality Assurance, Bharati Vidyapeeth’s College of Pharmacy, CBD Belapur, Navi Mumbai, Maharashtra 400614, India
E-mail: vineeta.khanvilkar@bvcop.in
| Date of Received | 02 August 2022 |
| Date of Revision | 12 March 2024 |
| Date of Acceptance | 21 May 2024 |
| Indian J Pharm Sci 2024;86(3):1138-1142 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Himalaya PartySmart capsule is a polyherbal formulation having liver-protective properties. The polyherbal capsule consists of extracts of six different herbs namely, Phoenix dactylifera, Cichorium intybus, Andrographis paniculata, Vitis vinifera, Phyllanthus amarus and Emblica officinalis. A reversed-phase high-performance liquid chromatography method with ultraviolet detection was developed to standardize commercial polyherbal capsule using andrographolide and catechin as markers. Two selected markers were separated on the C18 silica gel column by isocratic elution mode using methanol, phosphate buffer (potential of hydrogen 2.5) (70:30, v/v) as mobile phase at 27° and the detection wavelength was 259 nm. The method was validated as per the recommendations of the International Conference on Harmonization Q2 (R1) guidelines. Both the calibration curves exhibited a good linear coefficient of correlation (r2>0.995). The limit of detection and limit of quantitation values indicated that the developed method was sensitive even at low concentrations. The percent relative standard deviation of peak areas was less than 2 % for intra-day and inter-day precision. The recoveries of both markers ranged between 98 %-102 %. Two markers were quantified in polyherbal capsules containing andrographolide (0.72 %, w/w) and catechin (0.94 %, w/w). In conclusion, a specific, precise, accurate and robust reversed-phase high-performance liquid chromatography method was developed to determine andrographolide and catechin simultaneously which can be used to monitor the quality control studies.
Keywords
Andrographolide, catechin, simultaneous estimation, polyherbal formulation, method validation, high-performance liquid chromatography
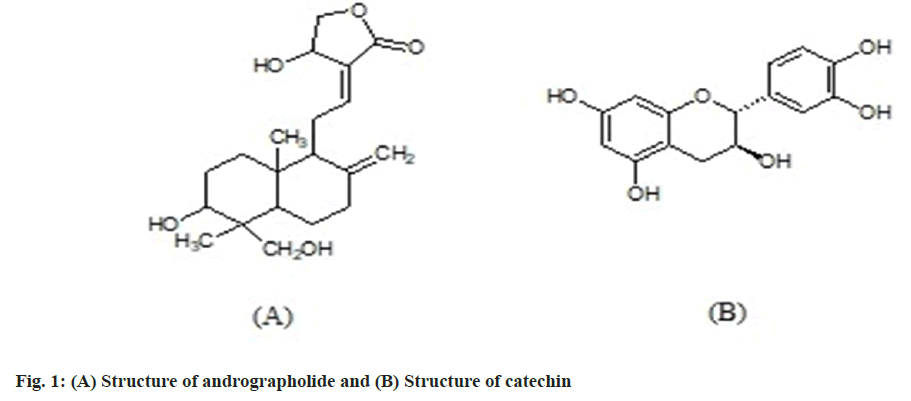
Herbs have been utilized to promote human health and well-being for ages. 'Return to Nature' is a term used to describe how mainstream modern medicine has begun to refocus on herbs and natural plant remedies as fundamental means of dealing with health issues. Approximately 80 % of the world's population uses herbal remedies. Herbal therapies are used two to three times more than conventional drugs around the world, according to the World Health Organization (WHO)[1]. As the commercialization of herbal medicine is widely increasing, assurance of the safety, quality and efficacy of herbal products has become an important issue[2]. Also, the complex nature and availability of phytoconstituents in variable amounts makes the process of standardization of herbal formulation more difficult. However, newer analytical techniques are coming to solve this issue with marker-based standardization as one of the significant methods. Identification of major and unique compounds in herbs as markers and the development of analytical methodologies for monitoring them are the key steps involved in marker-based standardization[3]. Various analytical methods like Thin-Layer Chromatography (TLC), High-Performance Thin Layer Chromatography (HPTLC), High- Performance Liquid Chromatography (HPLC), Ultraviolet Visible (UV) spectrophotometry, Liquid Chromatography-Mass Spectroscopy (LCMS), Liquid Chromatography-Nuclear Magnetic Resonance (LC-NMR) and Gas Chromatography- Mass Spectroscopy (GC-MS) are employed in marker based standardization. Due to sensitivity and accuracy, HPLC has gained importance in this process. Current research work focuses on the standardization of Himalaya PartySmart capsule, a polyherbal formulation reported to have liver-protective properties. It contains extracts of six different herbs namely, Phoenix dactylifera, Cichorium intybus, Andrographis paniculata, Vitis vinifera, Phyllanthus amarus and Emblica officinalis. Amongst them, Cichorium intybus, Andrographis paniculata and Phyllanthus amarus show hepatoprotective properties while Phoenix dactylifera and Vitis vinifera possess antioxidant properties. Emblica officinalis is reported to have hepatoprotective as well as antioxidant properties[4-6]. Extract of Andrographis paniculata is reported to be standardized using andrographolide as a marker by HPLC, HPTLC, TLC and UV methods[7-9]. Similarly, catechin quantification by the HPLC method[10] is reported for the standardization of Vitis vinifera extract. Considering this, we decided to select andrographolide (fig. 1A) and catechin (fig. 1B), as the markers for the standardization of polyherbal formulations containing these plants. Andrographolide (C20H30O5) has a molecular weight of 350.46 and is chemically known as 3,14,15,18-tetrahydroxy-5,9H,10-labda-8(20),12- dien-16-oic acid-lactone[11]. Andrographolide, which is a diterpenoid, is an active principle of Andrographis paniculata (Kalmegh), traditionally popular as a bitter tonic, febrifuge, blood purifier and natural hepatoprotective agent. Andrographolide is reported to have anti-inflammatory, anti-cancer, immunomodulatory, gastro and cardioprotective, analgesic, antipyretic, antibacterial, antiviral, antimalarial, antifertility, antihyperglycemic and hepatoprotective activities[12,13]. Catechin is a potent antioxidant and a polyphenolic component found naturally in a variety of plants. Catechin (C15H14O6) has a molecular weight of 290.27 and is chemically known as (2R-trans)-2-(3, 4-Dihydroxyphenyl)-3, 4-dihydro-2H-1-benzopyran-3, 5, 7-triol. Catechin is reported to have antibacterial, anti-allergic, anti-inflammatory, antiviral, anti-cancer and UV protection properties[14].
The literature survey showed reported methods for the estimation and quantification of andrographolide individually as well as in combination with other markers using HPTLC and HPLC[15,16]. Similarly, quantification of catechin individually as well as in combination with other markers using HPLC is reported[17]. However, no method was available for the simultaneous estimation of andrographolide and catechin using HPLC. The current research work was aimed at the development of a Reversed-Phase High-Performance Liquid Chromatography (RPHPLC) method for the simultaneous estimation of andrographolide and catechin. The method was validated as per the recommendations of the International Conference on Harmonization (ICH) Q2 (R1) guidelines[18]. Reference standards of andrographolide (purity≥98 %) and catechin (purity 92.2 %) were procured from Yucca Enterprises, Mumbai, and Maharashtra, India. HPLC grade methanol was purchased from SD Fine Chemicals Ltd., Mumbai, Maharashtra, India. The commercial formulation utilized in the study was purchased from a local pharmacy in Navi- Mumbai, Maharashtra, India. Chromatographic separation was obtained utilizing a reversed-phase Hemochrom C18 column (250 mm×4.6 mm ID, 5 μm pore size) with a Shimadzu Ultra-Fast Liquid Chromatography system (UFLC) (LC 2030) system, AutoSampler, SPD-20 a prominence Ultra Violet- Visible Spectroscopy (UV/VIS) detector. The data was collected using LabSolutions software and a mobile phase consisting of an isocratic mixture of methanol: phosphate buffer (pH 2.5) in a 70:30, v/v ratio. A 0.45 μm, 47 mm nylon 6, 6 membrane filter was used to filter the mobile phase. The injection volume was set to 10 μl, while the detection wavelength was set at 259 nm and the flow rate was maintained at 1.2 ml/min throughout the experiment. Column temperature was maintained at 25°. 10 mg of standard andrographolide and catechin was transferred individually in two different 10 ml volumetric flasks and volume was made up to 10 ml with methanol to obtain stock solutions having a concentration of 1000 μg/ml. These were stored in the refrigerator and used further to get 10-70 μg/ml solutions of andrographolide and catechin. Twenty capsules were opened and their content was transferred to the clean and dry petri dish. The average capsule content was determined by dividing the total weight by 20. The capsule content equivalent to 10 mg of Andrographis paniculata and Vitis vinifera was taken in a 10 ml volumetric flask. Then 5 ml of methanol was added and the mixture was sonicated for 30 min at room temperature to extract the selected markers. The volume was adjusted to the mark with methanol and was filtered through Whatman filter paper no. 40. This solution was then utilized for quantification. The working solutions of individual standards of andrographolide and catechin (10 μg/ml) were scanned in an Ultraviolet- Visible (UV-Vis) spectrophotometer in the range of 200-400 nm and then overlain spectra were obtained to determine an isobestic wavelength for subsequent HPLC analysis. Method validation was executed as per the recommendations of ICH Q2 (R1) guidelines for specificity, linearity, Limit of Detection (LOD), Limit of Quantitation (LOQ), precision, accuracy and robustness. Specificity was ensured by comparing the Retention time (Rt) of the standard markers with the marker components obtained in the chromatogram of the extracted capsule solution. Working solutions of 10-70 μg/ml of both standards were injected in triplicate to develop the calibration curves for andrographolide and catechin. The linearity of the method was determined by estimating the coefficient of correlation (r2), slope, and intercept of the calibration curves. LOD and LOQ were determined based on the standard deviation of response (σ) and the slope of the calibration curve (S) using the following formulas LOD=3.3 σ/S; LOQ=10 σ/S. For estimation of method precision, three different standard concentration levels were prepared, which contained 10 μg/ml (Lower Quality Control (LQC)), 40 μg/ml (middle quality control (MQC)), and 70 μg/ml (Higher Quality Control (HQC)) of andrographolide and catechin. The intraday precision was determined by analyzing these three working solutions in triplicate on a single day at three different times, whereas the interday precision was determined over 3 consecutive days. The percent Relative Standard Deviations (% RSD) were calculated. Standardization and quantification of andrographolide and catechin in the polyherbal capsule were accomplished using the developed approach. The amount of andrographolide and catechin present in the sample solution was calculated by using linear regression equation obtained from calibration curves of both the markers and the area observed from the sample solution. The result was then used for the recovery study. The accuracy was determined by spiking standard andrographolide and catechin solution to the marketed formulation at 80 %, 100 % and 120 % levels followed by analysis done in triplicate. Two concentrations namely LQC (10 μg/ml) and HQC (70 μg/ml) were analyzed in triplicate using the optimized chromatographic conditions with some deliberate changes.
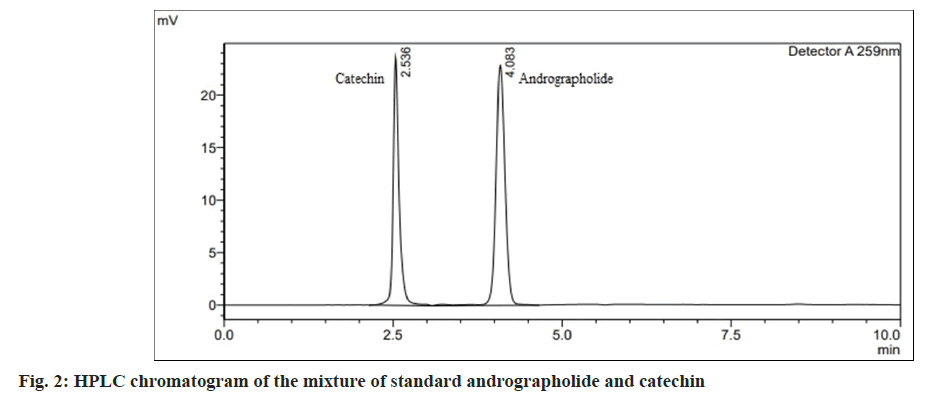
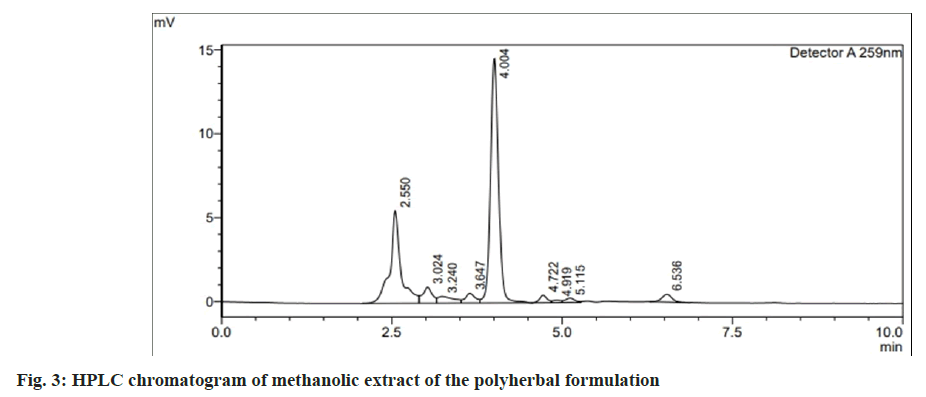
The overlain UV spectrum of andrographolide and catechin (10 μg/ml) showed an isobestic wavelength of 259 nm. This was chosen as the detection wavelength for HPLC analysis. Several mobile phase compositions were tried to optimize the RP-HPLC characteristics. A good resolution with good peak symmetry for andrographolide and catechin was obtained with a mobile phase containing methanol:phosphate buffer (pH 2.5) (70:30, v/v) at a flow rate of 1.2 ml/min. Rt of catechin and andrographolide was found to be 2.5±0.02 min and 4.0±0.02 min respectively, (fig. 2) shows the chromatogram of the mixture of standard andrographolide and catechin. Comparison of the chromatogram of the standard mixture of andrographolide and catechin and the extracted capsule sample showed no interfering peak at Rt of both the markers (fig. 3). The method was thus found to be specific for two selected markers. The method was found to be linear for andrographolide and catechin in the range of 10-70 μg/ml (Table 1). LOD and LOQ for andrographolide were found to be 8.5 μg/ml and 26.03 μg/ml respectively. LOD and LOQ for catechin were found to be 5.7 μg/ ml and 17.27 μg/ml respectively, which show the sensitivity of the proposed method even at low concentrations. The results of precision studies for andrographolide and catechin revealed that the method has good repeatability and reproducibility with a % RSD of less than 2 % for all concentrations (Table 1). Determination of accuracy at 80 %, 100 % and 120 % levels showed recovery within 98 %- 102 %, indicating the accuracy of the stated method (Table 1). Robustness was analyzed by making deliberate changes in chromatographic conditions including changing the content of organic solvent in the mobile phase (±1 ml of methanol), flow rate (±0.2 ml/min), and detection wavelength (±1 nm). The percentage RSD of the peak areas obtained for andrographolide and catechin was less than 3 %, which indicates the robustness of the method (Table 1). Quantification of markers andrographolide and catechin in the formulation was done by linear regression analysis. The content of andrographolide and catechin in the marketed polyherbal formulation was found to be 0.72 % w/w and 0.94 % w/w respectively. RP-HPLC method was developed for simultaneous estimation of andrographolide and catechin. The method was validated as per the recommendations of ICH Q2 (R1) guidelines and applied for the standardization of polyherbal formulation. This specific, precise, accurate, and robust RP-HPLC method could be employed in the quality control studies of the herbal formulations containing andrographolide and catechin as the major phytoconstituents.
| Parameters | Andrographolide | Catechin |
|---|---|---|
| Linearity range (μg/ml) | 10-70 | 10-70 |
| Regression equation | y=1807.3x+49668 | y=1551.6x+27128 |
| Coefficient of correlation (r2) | 0.9976 | 0.9989 |
| Precision | Precise | Precise |
| Accuracy | Accurate | Accurate |
| Robustness | Robust | Robust |
Table 1: Results of Method Validation Studies
Acknowledgments:
The authors would like to thank Bharati Vidyapeeth’s College of Pharmacy, CBD Belapur, Navi Mumbai, for providing the necessary facilities to perform this study
Conflict of interests:
The authors declared no conflict of interests.
References
- Pawar A, Rajalakshmi S, Mehta P, Shaikh K, Bothiraja C. Strategies for formulation development of andrographolide. RSC Adv 2016;6(73):69282-300.
- Bijauliya RK, Alok S, Chanchal DK, Kumar M. A comprehensive review on standardization of herbal drugs. Int J Pharm Sci Res 2017;8(9):3663-77.
- Ladva BJ, Mahida VM, Kantaria UD, Gokani RH. Marker based standardization of polyherbal formulation (SJT-DI-02) by high performance thin layer chromatography method. J Pharm Bioallied Sci 2014;6(3):213-9.
[Crossref] [Google Scholar] [PubMed]
- Indian Pharmacopoeia. 8th ed. Government of India, ministry of health and family welfare; 2018.
- Khare CP. Encyclopedia of Indian medicinal plants: Rational western therapy, ayurvedic and other traditional usage, botany. Springer. 2004.
- Hatem AA, Al-Khalifa AR, Amr AF, Mohamed MS. Effect of maturation stages on flavor profile and antioxidant activity of date palm fruits (Phoenix dactylifera) grown in Saudi Arabia. Int J Pharmacol 2018;14:407-14.
- Sajeeb BK, Kumar U, Halder S, Bachar SC. Identification and quantification of andrographolide from Andrographis paniculata (Burm. f.) Wall. ex Nees by RP-HPLC method and standardization of its market preparations. Dhaka Univ J Pharm Sci 2015;14(1):71-8.
- Misra H, Soni M, Mehta D, Mehta BK, Jain DC. An improved HPTLC-UV method for rapid estimation of andrographolide in Andrographis paniculata (Burm F.) Nees. In: Pharm Communique 2009;2(2):51-4.
- Ahamad J, Ameen MS, Anwer ET, Uthirapathy S, Porwal O. Qualitative and quantitative standardization of Andrographis paniculata by TLC technique and UV method. Adv Med Dent Health Sci 2019;2(3):29-32.
- Peng Z, Hayasaka Y, Iland PG, Sefton M, Hoj P, Waters EJ. Quantitative analysis of polymeric procyanidins (tannins) from grape (Vitis vinifera) seeds by reverse phase high-performance liquid chromatography. J Agric Food Chem 2001;49(1):26-31.
[Crossref] [Google Scholar] [PubMed]
- The merck index. 12th edition. Merck Research Laboratories, white house station; 1996.
[Crossref]
- Vetvicka V, Vannucci L. Biological properties of andrographolide, an active ingredient of Andrographis paniculata: A narrative review. Ann Transl Med 2021;9(14):1186.
[Crossref] [Google Scholar] [PubMed]
- Kishore V, Sastry YN, Bishayee A, Putta S, Malla R, Rao N, et al. Multi-targeting andrographolide and its natural analogs as potential therapeutic agents. Curr Top Med Chem 2017;17(8):845-57.
[Crossref] [Google Scholar] [PubMed]
- Bae J, Kim N, Shin Y, Kim SY, Kim YJ. Activity of catechins and their applications. Biomed Dermatol 2020;4:8:1-11.
- Jain V. Novel RP-HPLC method for simultaneous estimation of and rographolide and berberine in polyherbal formulation. Int J Ayurvedic Herb Med 2019;9(6):3679-86.
- Patel MB, Kadakia VM, Mishra SH. Simultaneous estimation of andrographolide and wedelolactone in herbal formulations. Indian J Pharm Sci 2008;70(5):683-89.
[Crossref] [Google Scholar] [PubMed]
- Kingori MS. Development of an improved isocratic HPLC method for the determination of gallic acid, caffeine and catechins in tea. J Nutr Health Food Sci 2018;6:1-9.
- Guideline IH. Validation of analytical procedures: Text and methodology. Q2 (R1). 2005;1(20):05.