- *Corresponding Author:
- Runjhun Tandon
Lovely Professional University, Jalandhar-Delhi G.T. Road, National Highway 1, Phagwara, Punjab-144 411, India
E-mail: gupta.runjhun@gmail.com
| Date of Submission | 25 February 2019 |
| Date of Revision | 12 May 2019 |
| Date of Acceptance | 11 September 2019 |
| Indian J Pharm Sci 2019;81(6):1127-1130 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The study of polymorphism in drugs is an important part of drug development process in present scenario. The present study identified a new polymorph of estradiol, which is one of the sleep awakening drugs in the market along with its characterization with powder X-ray diffraction, differential scanning calorimetry and thermogravimetric analysis and its stability data.
Keywords
Estradiol, polymorph, crystalline, amorphous, stability data
Polymorphism is obtained from Greek word, polus means many and morph, means shape. In other words, it is also defined as the ability of a substance to exist in two or more crystalline phases that have different arrangements or conformations of the molecules within a crystal lattice[1]. Different polymorphs of a drug substance have different physical properties like dissolution pattern, which in turn can affect their bioavailability and efficacy[2]. In addition, mechanical properties like hardness, tensile strength, flowability are also affected due to polymorphic change, which in turn affects the compatibility and hardness of a tablet or any other drug formulation[3]. Stability studies of the polymorph plays an important role for finalization of formulation process and storage conditions for a particular drug. For example, an amorphous form of atorvastatin calcium is unstable at higher temperature and its drug formulation is sold with a warning stating that the drug is to be stored at room temperature (25-30°)[4]. There are many drugs which show polymorphism with differences in their properties.
Literature survey revealed that only limited work has been done on polymorphic isolation of estradiol. Only 8 polymorphs have been reported till date (Table 1). It is widely used in combination with oral contraceptives and as a hormonal replacement therapy given in the form of transdermal patch. The patches being used are reported to crystallize after sometime, thereby affecting the uniform delivery of this steroid[5,6]. Different methods are reported in literature to get the desired polymorph using the antisolvent technique[7,8].
| Form Name | Characteristic pXRD peaks (2θ values) | TGA (In o) | DSC (In o) | Reference Number |
|---|---|---|---|---|
| Form G | 7.82, 8.91, 11.64, 13.22, 14.50, 15.30, 15.45, 15.60, 17.55, 18.20, 19.10, 20.01, 20.53, 21.92, 22.78, 23.93, 24.20, 26.14, 26.46, | 90-150 | 172, 177 | Present study |
| Hemihydrate | 11.71, 13.30, 14.61, 15.42, 15.61, 15.83, 17.77, 18.30, 19.11, 20.10, 20.53, 21.92, 22.70, 23.92, 24.22, 26.11, 26.66, | Not discussed | Not discussed | [10] |
| EM | 11.59, 13.17, 14.55, 15.51, 15.75, 17.67, 18.23, 19.05, 20.41, 21.72, 22.61, 23.80, 26.54 | 100, 155.6, 156.4, 170.67, 174.2 | 101.4, 154.8, 175 | [11] |
| ET | 13.26, 15.59, 15.87, 17.74, 18.33, 19.17, 20.52, 21.84, 22.71, 23.87, 26.66, 27.63 | Not discussed | Not discussed | |
| EP | 11.55, 13.12, 15.46, 15.69, 18.21, 19.00, 20.37, 21.70, 22.57, 23.75, 26.51 | Not discussed | Not discussed | |
| EC | 13.23, 15.54, 15.77, 17.69, 18.29, 19.01, 20.10, 20.45, 21.85, 22.69, 23.83, 26.59, 27.57 | Not discussed | Not discussed | |
| Anhydrous | 11.58, 13.18, 13.34, 17.72, 17.76, 19.15, 19.23, 19.19 | Not discussed | Not discussed | [12] |
| EA Hemihydrate | Not discussed | Not discussed | 110, 174 | [9] |
| ED (unstable form) | Not discussed | Not discussed | 169, 176-180 | |
| EC (Anhydrous) | Not discussed | No weight loss | 176-180 | |
| EM | Not discussed | 95-100 | 110-120, 145-160 |
PXRD is powder X-ray diffraction, TGA is thermogravimetric analysis and DSC is differential scanning calorimetry
Table 1: PXRD, TGA and DSC Values of Form G and Literature Reported Forms for Estradiol
In the present investigation, an attempt has been made to prepare a new polymorph of estradiol using solventantisolvent technique in which estradiol is dissolved in one solvent and an antisolvent was added to the resulting homogenous mixture to precipitate estradiol as the desired polymorph. Tert-butanol was purchased from Sigma Aldrich (Batch Number-360538) and estradiol (Batch No- 201006531) was obtained as a gift sample from Macleods Pharmaceutical Limited, India. Water was double distilled before using it as an antisolvent. A novel crystalline polymorph of estradiol was isolated using tert-butanol and water combination of solvents. Estradiol was dissolved in tert-butanol by heating up to 60° and water was added dropwise to precipitate estradiol as the desired polymorph. The new polymorph thus isolated was designated as form G in this study.
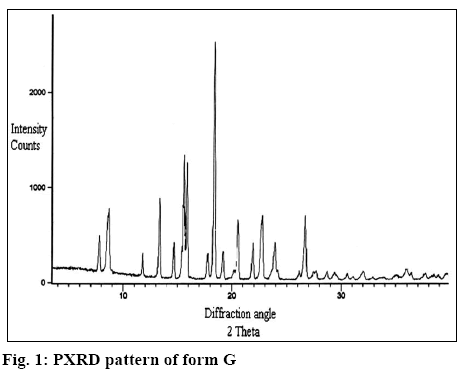
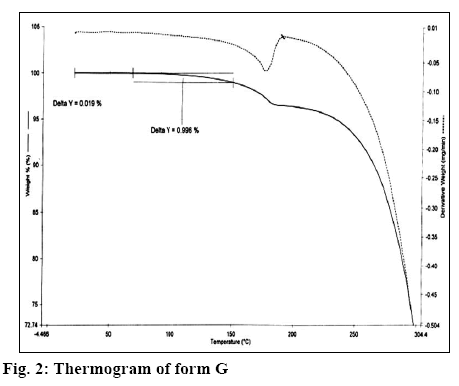
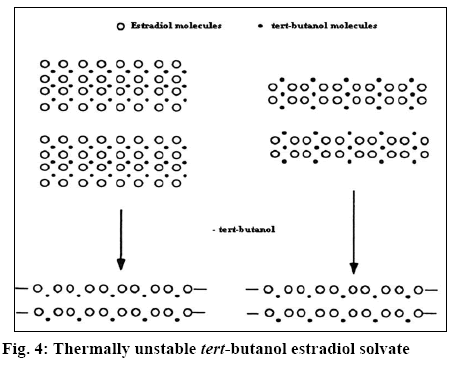
The X-ray diffractogram of form G is presented in figure 1. The diffractogram showed the presence of two additional peaks at 2θ 7.8 and 8.9. Thermal analysis is mainly used for identifying melting points of different crystalline forms of a compound and to assess the presence of a solvate as indicated by any weight loss during heating. Thermogravimetric analysis (TGA) of form G of estradiol reproduced in figure 2 showed a weight loss of 0.996 % between 90 to 150°, which is equivalent to 1 %. This could be due to the loss of tert-butanol from the tert-butanol solvate. The result is in consistence with the weight loss observed in case of the methanolate solvate of estradiol near 145°[9]. The form G exhibited thermal behaviour different from the hemihydrate form, which showed a weight loss of 3.2 % in the thermogram from 90-110°[10].
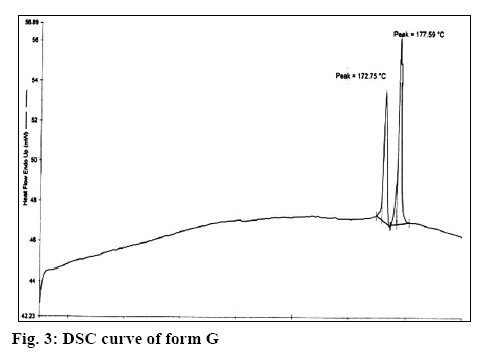
It is clear from Table 1 that Form G was different from all the other forms reported in literature. Form G has two characteristic peaks at 2θ values of 7.8 and 8.9 in powder X-ray diffractometry (pXRD). No peak at this region is reported for the hemihydrate[10], EM, ET, EP, EC[11] and anhydrous form[12], which clearly differentiated form G from these forms. This is further supported by the difference in the TGA and differential scanning calorimetry values (wherever reported as per Table 1, figure 3) for these forms. Further, as reported in literature[9], form ED is reported to be unstable and is always found as a mixture with EC form but stability study has shown that form G is stable indicating that form G is different from form ED. In addition, form EM shows 2 endotherm at 110-120° and 145-160° as compared to 2 endotherm at 172° and 177° in case of form G (figure 4) supporting the fact that form G is different from form EM.
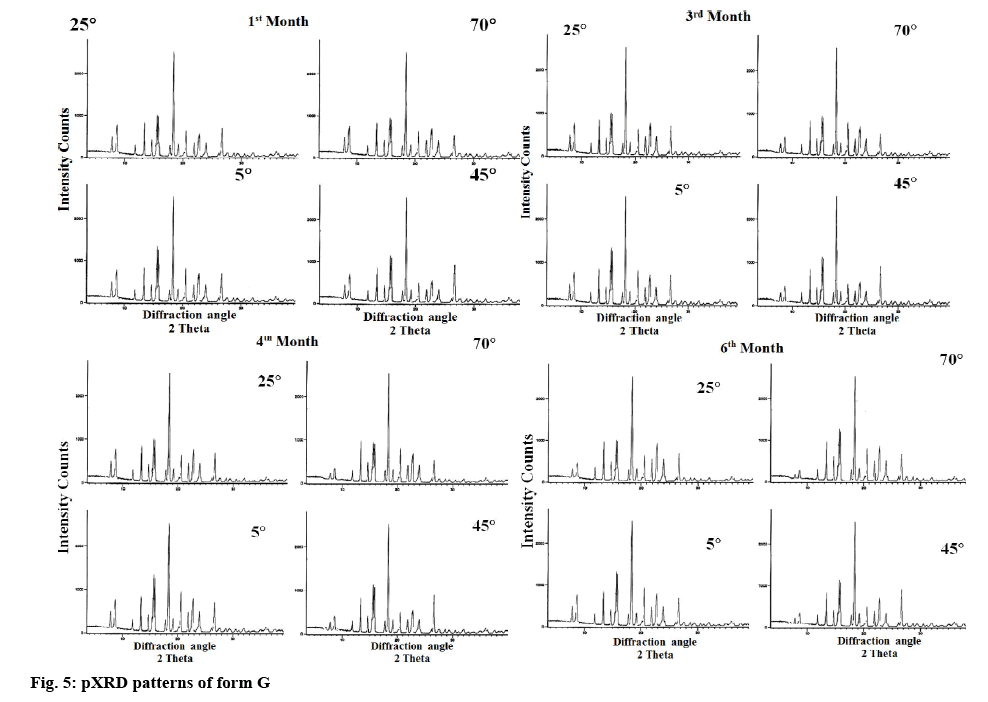
For the stability study of form G, the samples were packed in double polythene pack with aluminum foil and were stored for 6 mo at 5, 25, 45 and 70°. The pXRD’s of different samples were obtained after 1, 3, 4 and 6 mo. The pXRD’s of form G is reproduced in figure 5, and data are represented in Table 2.
| Time (mo) | 2θ values | Peak Intensity | |||
|---|---|---|---|---|---|
| 5o | 25o | 45o | 70o | ||
| 1 | No change | No change | No change | No change | |
| 3 | No change | No change | 7.8, 8.9 | 7.8, 8.9 | Decreased |
| 4 | No change | No change | 7.8, 8.9 | 7.8, 8.9 | Decreased |
| 6 | No change | 7.8, 8.9 | 7.8, 8.9 | 7.8, 8.9 | Decreased |
Table 2: Stability Data of Form G
Stability studies revealed that the new polymorph was stable at lower temperature but was converted into thermodynamically more stable hemihydrate form at higher temperature. Moreover, the rate of change of conversion from form G to hemihydrate form was found to increase with increase in temperature. The new polymorph was stable at all the temperatures studied and stable for 6 mo at 5°. However at 25°, the characteristic peaks at 2θ value of 7.8 and 8.9 were found to be diminished in the 6 mo sample indicating the initiation of conversion of form G into hemihydrate form. On the other hand at higher temperatures (45 and 70°) the characteristic peaks at 2θ value of 7.8 and 8.9 were found to diminish in the 3 mo sample itself. However 100 % conversion of form G to hemihydrate form was not observed even after 6 mo at higher temperature where still the mixture of hemihydrate form with a small amount of form G was observed as clear from the intensity of the characteristic peaks.
Acknowledgements:
Authors thank Lovely Professional University and Rajasthan University for providing the required facilities to carry out the work. Also, special thanks to Macleods Pharmaceutical Limited for providing estradiol as gift.
References
- Purohit R, Venugopalan P. Polymorphism: An Overview. Resonance 2009;14(9):882-93.
- Gupta KR, Karkar SS, Joshi RR, Padole YF. Solid State Properties: Preparation and Characterization. Der Pharm Sin 2015;6(4):45-64.
- Bernstein J. Conformational Polymorphism in Organic solid state chemistry. Amsterdam: Elsevier; 1987. p. 471-518.
- Shayanfar A, Jouyban A. Drug-drug coamorphous systems: Characterization and physicochemical properties of coamorphous atorvastatin with carvedilol and glibenclamide. J Pharm Innov 2013;8:218-28.
- Variankaval NE, Jacob KI, Dinh SM. Polymorphism of 17-beta estradiol in a transdermal drug delivery system. J Mater Sci Mater Med 2002;13(3):271-80.
- Variankaval NE, Jacob KI, Dinh SM. Crystallization of beta-estradiol in an acrylic transdermal drug delivery system. J Biomed Mater Res 1999;44(4):397-406.
- Tandon R, Tandon N, Gupta R, Gupta N. Art of synthesis of desired polymorphs. Asian J Chem 2018;30(1):5-14.
- Brittain HG. Polymorphism in Pharmaceutical Solids. New York, London: Informa Healthcare; 2009.
- Variankaval NE, Jacob KI, Dinh SM. Characterization of crystal forms of beta-estradiol, thermal analysis, Raman microscopy, X-ray analysis and solid state NMR. J Cryst Growth 2000;217:320-31.
- Kathleen CF, Jayalakshmi Y, Sussane ML, Pravin LS, inventor; Ortho McNeil Pharmaceutical Inc, assignee. Crystalline form of estradiol and pharmaceutical formulations comprising same. United States patent US5928666. 1999 July 27.
- Park JS, Kang HW, Park SJ, Kim CK. Use of CP/MAS solid-state NMR for the characterization of solvate molecules within estradiol crystal forms. Eur J Pharm Biopharm 2005;60:407-12.
- Stevenson EL, Lancaster RW, Buanz AB, Price LS, Tocher DA, Price SL. The solid forms of sex hormone 17-beta estradiol. Cryst Eng Comm 2019; 21:2154-63.