- *Corresponding Author:
- Shivanjali Madane
Department of Pharmaceutics, PES Modern College of Pharmacy (For Ladies), Moshi, Maharashtra 412105, India
E-mail: madaneshivanjali6@gmail.com
| Date of Received | 26 December 2022 |
| Date of Revision | 24 April 2023 |
| Date of Acceptance | 11 June 2024 |
| Indian J Pharm Sci 2024;86(3):791-804 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Micelles are loosely bound aggregation of several tens or hundreds of atoms, ions or molecules, forming a colloidal particle. Micelles can either passively or actively target their payload to particular tissues in addition to dissolving hydrophobic medicines. Micelles nano-carriers hold the promise of solving the drug solubility issue among the various drug carriers that have been created. Gels are homogenous, semisolid preparations that often include one or more medications in the form of solutions or dispersions in appropriate hydrophilic or hydrophobic base. Now-a-days micellar gel can become promising approach for enhancing drug permeation through skin. Micellar gel shows higher penetration for treating deeply situated infections (example fungal infection). Micellar gel is generally prepared to increase the permeability or penetration of the medication across the skin. Micellar gels mainly used for providing hydrophobic medication which are encapsulated within micelles and deliver it to specific site. These benefits of micellar microparticles open up a lot of potential for the topical distribution of hydrophobic drugs in the future, with increased efficacy and lower production costs. There are several methods used for micelles and gel preparation such as dialysis method, solid dispersion method, microphase separation method, solvent evaporation method, thermal change method, chemical reaction and flocculation method. The characterization for micellar gel is particle size, critical micelles concentration, drug entrapment efficiency, drug content, nuclear magnetic resonance, differential scanning calorimetry, viscosity, spreadability, extrudability, patch test, skin irritation test and ex vivo skin permeability test.
Keywords
Gel, micellar gel, micelles, polymeric micelles
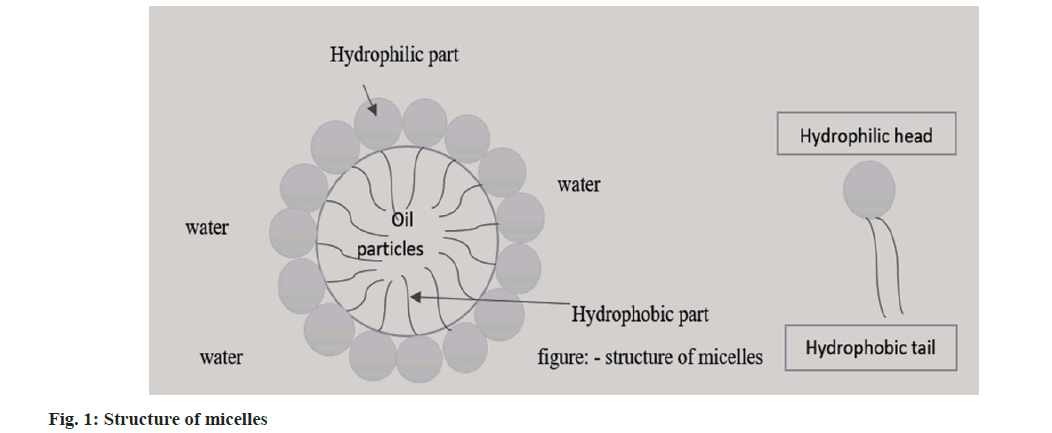
The hydrophobic property of pharmaceuticals, which is connected to ever-increasing solubility issues for the past 10 y is the main issue facing contemporary pharmaceutical research. At this time, 70 % or more of potential novel drugs are insoluble in organic and aqueous media. Furthermore, 40 % of commercially available oral medicines are regarded as hydrophobic medications. To achieve therapeutic pharmacological effects in the blood, it is required to consider raising the drug dose. Traditionally, nano-sized carriers such as liposomes, niosomes, micelles, emulsions, etc., were used to encapsulate hydrophobic medicines. Even while some of these carriers (like liposome and noisome) have the potential to increase the solubility of hydrophobic drugs, some of them are unable to contain the hydrophobic molecules. Micelles nano-carriers therefore hold the promise of solving the drug solubility issue among the various drug carriers that have been created or are being researcher[1]. However, there is relatively little information available regarding the toxicity of polymeric-micelle carriers, primarily because the toxicity of pharmaceuticals that are not targeted is typically much more severe than that of carriers used in anticancer drug targeting[2]. Block copolymer micelles can either passively or actively target their payload to particular tissues in addition to dissolving hydrophobic medicine[3]. For the solubilization of lipophilic drugs in micelles, which typically have a diameter of 10-100 nm, an inner core made up of the hydrophobic sections of the amphiphiles produces an outer space. A palisade or corona made of the hydrophilic building blocks of the amphiphiles encircles the core region. These conformations sterically inhibit the opsonization of blood constituents, preventing macrophage phagocytosis, reducing reticuloendothelial system clearance and prolonging the period of circulation[4]. The structure of micelles is shown in fig. 1. According to the immunoprecipitation, gels are homogenous, semisolid preparations that often include one or more medications in the form of solutions or dispersions in appropriate hydrophilic or hydrophobic bases[5]. Drug gradient in the gel layer is a factor in delivery kinetics. Thus, drug flux is controlled by drug concentration and gel layer thickness. The amount of medication and its solubility in the gel is related[6]. Gels are used to administer a topical medication that has been rubbed onto the skin, the eye or the mucous membrane. Shampoos, colognes, toothpaste, skin and hair care products and other cosmetics[7].
Micellar Gel
The polymeric materials promise a variety of future uses, the potential to modify their properties over a wide range has attracted a lot of attention[8]. Over the past few decades, numerous scientists have worked to create strong hydrogels and organogels in an effort to solve this issue[9]. Large concentrations of solvent typically result in gels with poor mechanical characteristics. In various publications, the topical delivery of medications into the deep layers of the skin via micelles has been described as a potential means of getting around the limitations of the systems mentioned above. The transport of the therapeutic agent to the target compartment is successfully ensured by topical drug administration via micellar system and the possibility of systemic adverse effects is decreased. Drugs that are hydrophobic become more soluble and permeable due to the active medicinal ingredient's micellization. Many dynamic supramolecular structures, including polymeric micelles have been developed in recent years as a result of advances in supramolecular chemistry. Because they have a lower critical micelle concentration than surfactant-derived micelles, Pluronic’s, which are block copolymers based on ethylene oxide and propylene oxide can create more stable polymeric micelles. Monomers and micelles coexist above the Critical Micelles Concentration (CMC) in a dynamic equilibrium. A wide range of medications that are difficult to dissolve can be solubilized by polymeric micelles due to their great stability in vitro and in vivo and strong biocompatibility. There are numerous drug-loaded micelles undergoing various stages of preclinical and clinical testing at the moment. The hydrophobicity of the micelles' core and bridging blocks determines how long the crosslinks (bridges) in physically bonded micellar gels can stay inside the micelles. Rheometrics was used to assess the viscoelastic and gelation kinetics of physically and covalently crosslinked micellar gels. The micellar gel precursor solutions retained a viscous response at low frequencies and an elastic response at high frequencies, according to the results of frequency sweep tests on physically crosslinked micellar gels at temperatures just above the sol-gel transition[10].
For better foam stability and detergency, viscoelastic worm-like micellar solutions are added to detergents and personal care items like shampoo. Because they have a minimal impact on the environment and gentle on the skin, wormlike micellar compositions employing surfactants from renewable resources are in demand[11]. Rheology allowed us to investigate the mechanical characteristics of the micellar gels produced in the concentrated regime, while light scattering was previously utilised to identify the usual sizes of those micelles in the dilute regime. However, there is currently no information available regarding the micelles' internal structure or how they are organised when they are in the gel state[12].
The first amount of medicine added affects how well a drug is incorporated. Drug precipitates once it has loaded to its maximum capacity. By designing the polymeric core to improve the interaction of the drug with the micelle core, drug release from polymeric micelles can be controlled. There are two main methods that pharmaceuticals are released from the micellar core, either the micelle is dissociated followed by the drug's separation from its monomers or the drug-polymer link breaks within the micelle and is then diffused out of the delivery system[13].
Advantages of micellar gel:
Micellar gels offer several advantages in drug delivery systems. They enable the targeted delivery of medicine to specific sites and allow the encapsulation of hydrophobic medications. Many drugs have high lipophilic character micelles are an effective carrier system for delivering both the hydrophilic and lipophilic drug in tissues. These gels can be produced quickly and cheaply and they have the ability to release molecules gradually over time while circulating in the bloodstream, enhancing penetration capacity compared to other dosage forms[14,15] thereby achieving more penetration capacity than other dosage form. These gels can be produced quickly, cheaply and they have the ability to release molecules gradually over time while circulating in the bloodstream, enhancing penetration capacity compared to other dosage forms. Micellar gels avoid digestive compatibility issues and are convenient and simple to apply offering a larger application area than the buccal cavity, which increases patient compliance. They eliminate the risks and drawbacks associated with intravenous therapy and various absorption-related factors such as pH changes, the presence of enzymes and gastric emptying time. By continuously releasing the medicine, they achieve efficacy with a lower total daily dose and enable precise distribution of medications to particular locations, making them suitable for self-medication.
Disadvantages of micellar gel:
Micellar gels also come with some disadvantages to consider. Firstly, measurement of non-spherical micelles with poor precision and accuracy[16], the influence of temperature, humidity and other environmental factors can change the rheology of some gels[17] and drugs that cause skin irritation or sensitization should not be administered via this route[18].
Mechanism of micllelar gel:
Researchers recently revealed a simple approach for making self-healing hydrogels that uses locally produced hydrophilic polymers that have undergone hydrophobic modification along with watchlist management operating in the semi dilute regime. Micellar copolymerization produces dynamic hydrophobic connections between the hydrophobic domains of polymer chains and surfactant micelles, which serve as physical cross-links of the resultant gels. Rapid self-healing of the hydrogels without the requirement for any stimulus or healing agent is achieved by reversible breakable cross-links. The hydrogels gained interest in recent years due to their distinctive properties, such as their insolubility in water but solubility in surfactant solutions, nonergodicity and extreme toughness[19]. However, the mechanism of self-healing and the evolution of the nanoscale structure during the production of micellar hydrogels are still not fully understood. The monomers are transformed into hydrophobically modified hydrophilic polymer chains during micellar polymerization, resulting in a hydrogel with mixed micelles acting as physical cross-links[20].
Topical Drug Delivery System
For the purpose of treating local disorders, the term "topical delivery system" refers to a technique in which the formulation is applied to the skin, eyes, nose and vagina. The first pass metabolism of the liver, changes in gastric pH, and fluctuations in plasma levels that are typically experienced when a drug is delivered orally are avoided when the substance is applied to the topical surfaces. For example, voclosporin (0.2 % w/v) loaded nano micellar formulations were created and their ocular distribution and pharmacokinetics were evaluated by installing single and repeat dosage studies in rabbits. Rapid medication penetration was achieved with just one dose of the topical formulation. Due to their improved drug bioavailability and better capacity to pass through ocular epithelia, micelles are becoming increasingly important in the administration of ocular drugs[21].
Methods of Formulation of Micellar Gel
The various method of formulation of micellar gel is discussed in fig. 2, which is given below.
Micelles formation:
Dialysis method: The dialysis approach, which used free surfactant was used to create polymeric micelles. Di block copolymers were dissolved in solvent, added to a dialysis tube and dialyzed against 1 l of distilled water over the course of 24 h. The suspension was freeze dried after being filtered using a 0.45 μm filter to remove aggregates (fig. 2)[22].
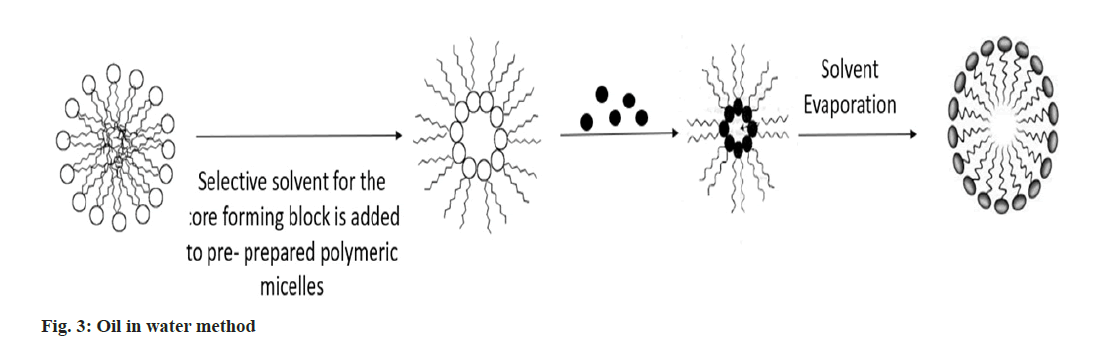
Oil in water method: Drug was dissolved into chloroform, drug-free polymeric micelles were dissolved into distilled water and homogenised for 30 s using a sonicater. The drug's chloroform solution was stirred vigorously and dropwise into the polymeric micelle solution in distilled water. To remove chloroform by evaporation, the reaction mixture was agitated in an opens air system. After that, the mixture was lyophilized after being ultrafiltered on an Amicon YM-30 membrane to get rid of any remaining unbound drugs and impurities (fig. 3)[23].
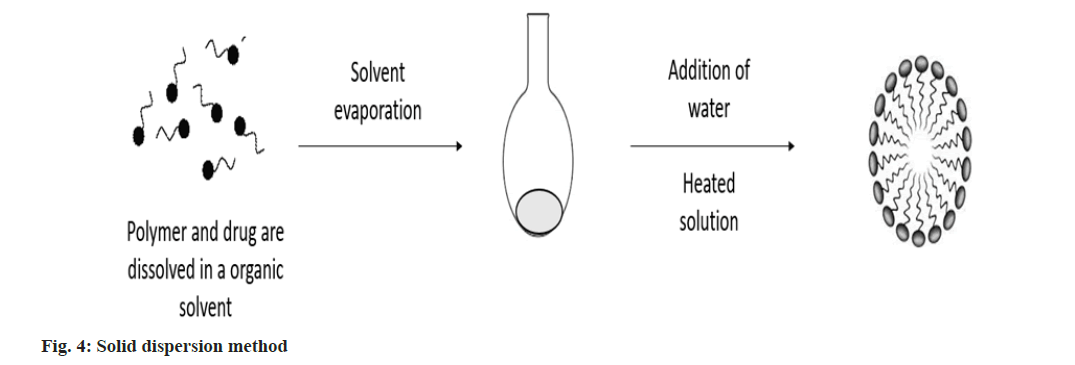
Solid dispersion method: This approach involves dissolving the drug and polymer together in an organic solvent, which is then evaporated under decreased pressure to produce a solid polymer matrix. Water is added to the heated polymer matrix to produce drug-loaded polymeric micelles (fig. 4)[24].
Microphase separation method: This technique involves dissolving the medication and the polymer in tetrahydrofuran, an organic solvent and adding the solution dropwise while magnetic stirring is applied to the water. Drugs are included in the inner portion of the polymeric micelles that spontaneously form under lower pressure, the organic solvent is eliminated and a blue-colored polymeric micelles solution is created[25].
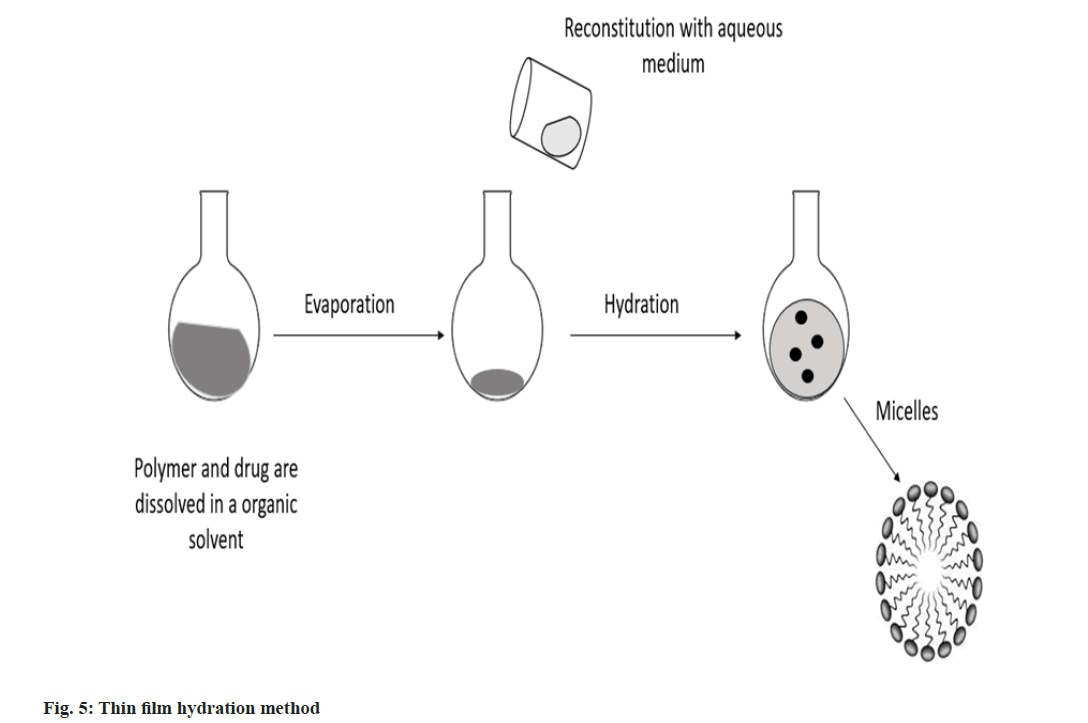
Thin film hydration method: In an organic solvent, copolymer, triethylamine and medication solution were dissolved and then combined for 1 h. A drug containing lipid membrane was created by first removing the organic solvent using rotary vacuum evaporation, followed by an additional 30 min of drying under a continuous nitrogen flow. The drug-containing lipid membrane was dissolved in 1 ml of pH 7.4 buffer solution, heated and swirled to create a draught nano micelle solution. After centrifuging this nano micelle solution for 20 min at 13 000 rpm, the final nano micelle was obtained by filtering through 0.22 mm filters (fig. 5)[26].
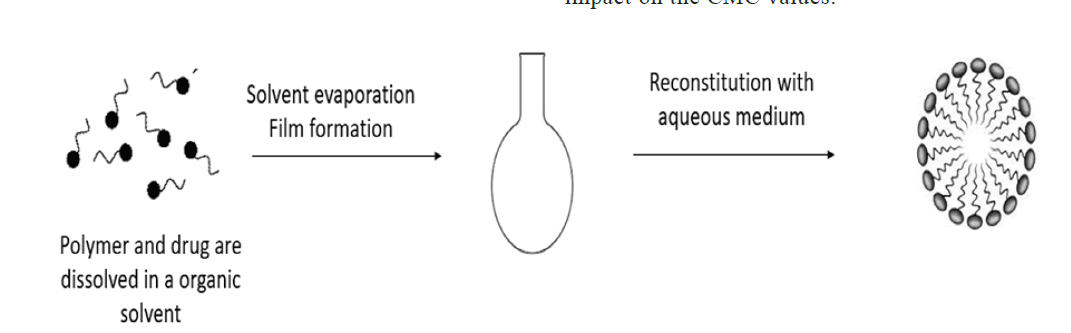
Solvent evaporation method: Copolymer was typically dissolved in a solvent (ethanol or an ethanol/chloroform combination), which was then vigorously stirred into distilled water to cause the production of micelles. In order to evaporate the solvent and create the "core-shell" blank micelles, the solution was exposed to the air and stirred continuously all night (fig. 6)[27].
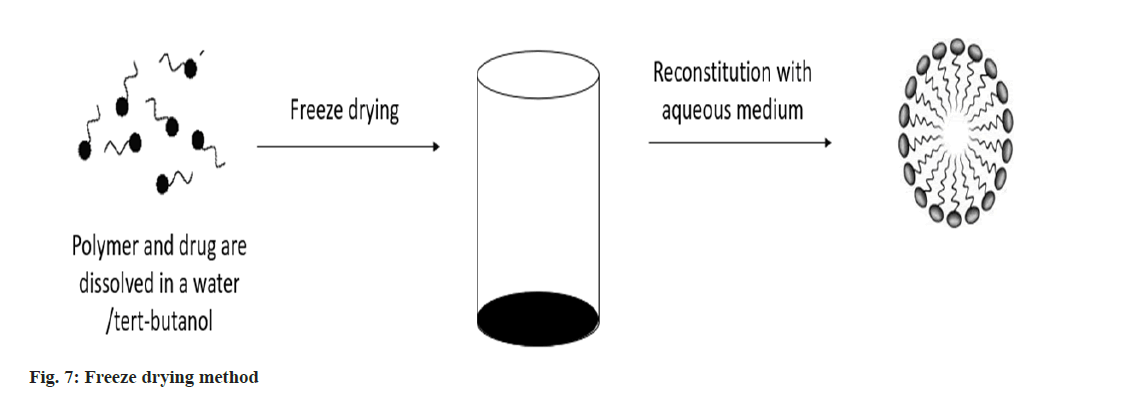
Freeze drying: The polymer and medicine are dissolved using the freeze-drying technique using a freeze-dryable organic solvent, such as tertbutanol. Following the addition of water, this solution is freeze dried and reconstituted using isotonic aqueous media. This approach is only applicable to block copolymers and drug structures that can be solubilized in tert-butanol, even if it may be pharmaceutically practical for large-scale manufacture (fig. 7)[28].
Methods of preparation of gel:
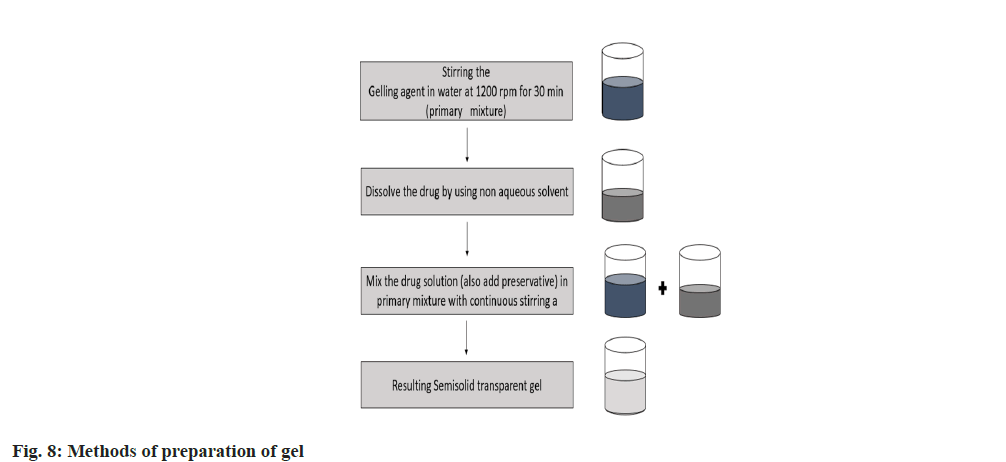
The simple method for preparation of gel is discusses in fig. 8, which is given below.
Thermal change method: Cellulose derivatives, gelatin, agar sodium oleate, guar gum, etc. Some substances, such as cellulose ether, on the other hand, have their water solubility due to hydrogen bonding with the water. These solutions lower solubility and broken hydrogen bonds will result in gelation as the temperature is raised. As a result, this technique cannot be used to create gels in general[29].
Flocculation method: Here, gelation is created by adding just the right amount of salt to cause age state precipitation, but not enough to cause full precipitation. In order to prevent a localised high concentration of precipitant, fast mixing must be ensured. For instance, ethyl cellulose and polystyrene solutions in benzene can be quickly mixed with the appropriate proportions of a non-solvent, like petroleum ether. Salts cause coagulation and gelation when added to hydrophobic solutions, respectively. The gels created using the flocculation process behave in a thixotropic way[30].
Chemical reaction method: By a chemical reaction involving the solvent and solute, gel is created in this process. An increased concentration of the reactants will result in a gel structure, as in the case of the formation of aluminium hydroxide gel by interaction of an aluminium salt and sodium carbonate in an aqueous solution. There are a few other cases where the polymeric chain is crosslinked chemically through the reaction of PVA, cyanoacrylates and glycidol ether with toluene diisocyanates, methane diphenyl isocyanine and Tolulene Diisocyanates (TDI)[31].
Complex conservation method:
By combining a polyanion and a polycation, complex coacervate gels can be produced. The fundamental idea behind this procedure is that polymers with opposite charges attract one another and can combine to produce soluble or insoluble complexes, depending on the concentration and pH of the corresponding solutions. For example, combining polycationic chitosan and polyanionic xanthan is one instance of this. Positively charged proteins below their isoelectric point are likely to combine with anionic hydrocolloids to create poly ion complex hydrogels[32].
Factor Affecting Micellar Gel
Thermodynamic and kinetic factors influencing stability:
Polymeric micelles stability can be separated into thermodynamic and kinetic stability, both of which are necessary for in vivo drug administration. The polymer chains in micelles are still engaged in a dynamic exchange with those in the medium. To prevent the encapsulated drug cargo from releasing too soon, it is crucial to describe the parameters that affect the stability of micelles. According to reports, the glass transition temperature (Tg) of hydrophobic blocks (or the condition of the micelle core) can significantly affect thermodynamic and kinetic stability, which in turn affects drug release and the pharmacokinetics behaviour of the drug in polymeric micelles.
Critical micelle concentration of polymer:
The tendency for aggregation and thermodynamic stability of Polymer Micelles (PMs) selfassembled by amphiphilic block copolymers with lower CMC are stronger than those of micelles generated by low molecular weight surfactants. Additionally, because the CMC is linked to the standard free energy and the free energy, both of which are useful to the exchange behaviour between micelles and polymer chains, the CMC of polymers may have an impact on the kinetic stability of PMs. Micelles that self-assemble using polymers with lower CMC typically have higher kinetic stability. The hydrophobic/hydrophilic ratio and interactions between polymers and the environment, such as temperature, pH, and salt concentration parameters, all have an impact on the CMC value.
Ratio of hydrophobicity and hydrophilicity:
The CMC values are mostly influenced by the polymer’s hydrophobicity and they decrease as hydrophobicity increases, indicating high stability for PMs. The length of the hydrophobic block can be increased, increasing the polymers' hydrophobicity. The ratio of a copolymer's hydrophilicity to hydrophobicity may alter depending on the length of the hydrophilic block, which may also have an impact on the CMC values.
Interaction between polymers: The contacts between polymers, which are essential for the interconversion between micelles and free polymer chains, aid in the production of PMs and preserve their structural stability in vivo. These interactions include hydrophobic, electrostatic/ionic, stereo complexation, and hydrogen bonding. As an example, consider hydrogen-bonding compounds where two nucleic acids are enclosed by block copolymers.
Environmental factors: CMC and the size of micelles may be impacted by environmental factors such temperature, salt type or concentration, and pH. The formation of micelles is a temperaturedependent process and even a small rise in temperature may cause a sharp drop in the CMC. The Poly(Ethylene Oxide)-Poly(Propylene Oxide)- Poly(Ethylene Oxide) (PEO-PPO-PEO) block copolymers have been found to produce micelles in water with a strong temperature dependence[33].
Gel microstructure: The three-dimensional gel structure's porosity, which affects how quickly pharmaceuticals diffuse into the environment, is heavily influenced by the amount, size and arrangement of micelles.
Mechanical and Rheological Properties
The release profile can be maintained by improving these qualities since they can significantly lower gel erosion and drug diffusion rates. For example, poloxamer-based in situ gels frequently have extremely porous structures with connected water channels that hasten the erosion of the gel matrix[34].
Evaluation Parameteres or Characterization of Micellar Gel
Particle size, zeta potential and polydispersibility index:
The size and size distribution of the total amount of drug entrapped in the drug-loaded polymeric micelles were measured after they had been created by film sonication. Laser light scattering was used to determine the composition of the polymeric micelles (Zetasizer Nano ZS, Malvern)[35].
Morphological evolution:
Transmission Electron Microscopy (TEM) was used to observe the morphology of the medication[36].
pH determination:
A digital pH metre was used to measure the pH of micellar gel compositions. After dissolving 1 g of gel in 100 ml of distilled water, it was left to stand for 2 h each formulation's pH was measured three times and using a pH metre, the average results were computed[37].
Grittiness and homogeneity:
All gel preparations must meet the requirements for freedom from specific matter and grittiness as needed for topical applications. Gel formulations were analysed microscopically to look at the particulate debris that was visible under a light microscope. Visual inspection was used to check the homogeneity of all manufactured gels. The appearance and existence of any aggregates in the gels are evaluated[38].
Critical micelle concentration:
By employing pyrene as a fluorescence probe, fluorescence spectroscopy was used to quantify the CMC of the mixed micelles. Pyrene would travel inside micelles from the aqueous phase upon micelle formation, changing the intensity ratio of I372/I383. At room temperature, F-2500 fluorescence spectrophotometer was used to record the fluorescence spectrum. By dilution drug mixed copolymer in distilled water, solutions of various concentrations of drug mixed copolymers were created. Pyrene and the mixed copolymer solution were combined and then the mixture was left to equilibrate for 24 h at room temperature[39].
Determination of drug content in micelles:
Drug-loaded micelles were suitably dissolved in acetonitrile and vortexed to produce a clear solution and then the concentration of the drug was determined using High Performance Liquid Chromatography (HPLC).
Percentage drug concentration=weight of the drug within micelles/weight of the feeding polymer and drug×100 %[39].
Differential Scanning Colorimetry (DSC):
DSC is used to assess the nature and speciation of the crystallinity within micelles. DSC is used to determine the different states of PMs' cores as well as the degree of interactions between drugs and polymers DSC experiments were carried out to verify that the medication was indeed trapped inside the micelles[40].
HPLC analysis:
The drug's concentration was determined using HPLC. A 0.22 ml membrane filter was used to filter the mobile phase before it was produced and eluted at a rate of 1 ml/min. The drug concentration was calculated using injection volume at 70° and a UV detector set at 210 nm[41].
Entrapment efficacy:
A test tube containing the drug formulation was filled with 10 ml of Phosphate-Buffered Saline (PBS) (pH 7.4). This liquid was subjected to a 10 min sonication in a sonicater bath. Centrifugation was used to separate the drug-containing solution from the unentrapped drug for 30 min at 20° at 25 000 rpm. After being taken out, the supernatant was diluted with PBS. The UV spectroscopic approach was used to measure the drug concentration in the resultant solution. The following equation was used to determine the percentage of medication encapsulation,
EP (%)=[(Ct-Cr)/Ct]×100.
Where, EP is the Encapsulation Percentage, Ct is the concentration of entire drug and Cr is the concentration of free drug[42].
Viscosity:
Micellar gel's viscosity was measured using a Brookfield viscometer[43].
Spreadability:
Using spreadability apparatus, the spreadability of micellar gel formulations was evaluated. A lower slide was used to hold 1.0 g of the micellar gel sample and upper slide as used to cover it. The formula was used to calculate the spreadability[44].
Skin irritation test:
Animal skin, specifically either gender of guinea pig (400-500 g), was used for the skin irritancy test for micellar gel. The hair on the animal's back was trimmed before the micellar gel was applied to a 4 cm2 of the surface. The guinea pig's skin was exposed to the micellar gel twice daily for 7 d. The site was examined for sensitivity and reaction and it was graded as 0, 1, 2, or 3 for no reaction, light patchy erythema, light but confluent erythema, moderate but patchy erythema and severe erythema without or with edema respectively[45].
Physical stability:
Samples of the formulations were obtained at 1, 7, 14 and 90 d following 3 m of storage at 4°. HPLCfluorescence was used to evaluate the amount of drug in the formulations of micelles in order to determine how much of it is present[46].
Extrudability test:
Standard collapsible aluminium tubes with caps were filled with the micellar gel compositions and the ends were crimped shut to seal. The tubes weights were recorded. The tubes were placed between two glass slides and then clamped. The cap was removed after 500 g of material had been applied to the slides. We measured the volume of the extruded micellar gel. Calculated extrudability percentages are as follows; >90 % extrudability=excellent, >80 % extrudability=good and >70 % extrudability=fair[47].
in vitro drug release:
The dialysis approach was used in a dialysis bag, 1 ml of polymeric micelles containing drug was placed. Then, a release chamber holding 500 ml of pH 7.4 PBS was filled with the firmly packed dialysis bag. The experiment was carried out at 37° with 100 rpm magnetic stirring that was ongoing. 1 ml of the sample was taken out and immediately replaced with an equal volume of fresh release medium at predetermined intervals. The amount of drug in the sample was determined using HPLC. For comparison, a release study of the medication solution was also carried out[48].
Ex vivo skin permeability test:
A transdermal membrane made of guinea pig abdominal skin was used in ex vivo skin permeability testing utilising Franz diffusion cells. Hair on the guinea pigs' abdomens was trimmed with an electric razor. The guinea pigs were gently narcotized with diethyl ether and then a 1.5 cm diameter circular area of abdominal skin was removed using a corneal trephine. The wounds were then sutured and sterilised. The donor chamber was placed between the receiver chamber, which held 5 ml of PBS and the abdomen skin. The donor chamber was in front of the stratum corneum. In the receptor chamber, the PBS was stirred by a magnetic bead (300 rpm). The diffusion cells were kept at a temperature of 37°. By using HPLC, the medication concentrations in PBS solutions were examined. Plots depicting the cumulative drug concentrations per unit area in the receiver chamber as a function of time were made. The slope of linear part of the line was used to determine the steady-state flux[49].
Statistical analysis:
Data are shown as the mean and standard deviation. The release study's results were statistically analysed using student's t-test[50].
Marketed Preparation of Micellar Gels
Recently many researchers have worked on micellar gel for various diseases like fungal, gynaecological, ocular diseases, psoriasis. But micellar gel has not yet come in the market for medicated purposes. Table 1, provides information on marketed preparations of micellar gel, while Table 2, presents details on research work conducted in various areas involving micellar gel[51-77].
| S. no | Marketed brand name | Uses |
|---|---|---|
| 1 | Micellair skin breathe (NIVEA) | Make up remover wash gel |
| 2 | ACO (sensitive micellar gel) | Cleansing face and eye |
| 3 | Micellar gel wash (Dr. Vanitha rattan) | Controlling sebum and skin colour |
| 4 | Aveeno (positively radiant) | Draw out skin dulling impurities |
| 5 | Babe micellar gel | Cleansing or soothing activity for all type skin |
| 6 | Simple water boosts micellar facial gel wash | For removing dirt and make up |
| Instantly restore hydration to dehydrated skin | ||
| 7 | Bioderma sensibio gentle soothing micellar foaming gel | Used for cleansing sensitive skin face and eye |
Table 1: Marketed Formulations Of Micellar Gel
| S. no | Drug | Uses | Outcome | References |
|---|---|---|---|---|
| 1 | Docetaxel | Breast cancer and ovarian cancer | Formulation having good stabilizing property and also increased the anti-tumour activity | [51] |
| 2 | Terbinafine hydrochloride | Onychomycosis | This micellar gel deeper areas of hooves for dermatophytes | [52] |
| 3 | Resveratrol | Psoriasis | This formulation treating Plaque psoriasis with superior dermatological outcomes | [53] |
| 4 | Paclitaxel | Cutaneous malignancy | local chemotherapy is possible of PTX-micellar gel and inhibited the growth of the melanoma in vivo | [54] |
| 5 | Curcumin | Eye diseases | These results demonstrated the biocompatible and excellent potential for effective ophthalmic drug therapy | [55] |
| 6 | Ibuprofen (IBU) and Ibuprofen Sodium Salt | Analgesic, anti-inflammatory | This formulation increases the therapeutic efficacy through the simultaneous delivery of these agents to a target tissue or organ | [56] |
| 7 | Simvastatin | Wound healing | This micellar gel having a good wound healing activity | [57] |
| 8 | Curcumin | Ocular diseases | Formulation shows increased the solubility, stability, corneal permeability and no irritant effects on the ocular tissues | [58] |
| 9 | Naproxen and indomethacin | Anti-inflammatory | It shows stronger effects partitioning and good drug-water hydrophobic interactions | [59] |
| 10 | Paclitaxel (PTX) | Anticancer | The formulation shows good stability and solubility | [60] |
| 11 | Miconazole nitrate | Deep fungal diseases | This formulation shows greater permeability and it is more effective at treating ingrained infections | [61] |
| 12 | Lurasidone hydrochloride | Bipolar disorders and schizophrenia treatments | It shows prolonged release with improved permeability and brain bioavailability | [62] |
| 13 | Griseofulvin and fluconazole | Fungal diseases | It improves the bioavailability and preventing negative side effects than oral dosage form | [63] |
| 14 | Doxorubicin and hydrochloride | Anticancer | This micellar gel improving permeability and patient compliance by avoiding repeated drug administration. | [64] |
| 15 | Ciprofloxacin and hydrochloride | Ocular diseases | This formulation shows prolonged precorneal drug release, improved ocular bioavailability, required fewer administrations, and hence increased patient compliance | [65] |
| 16 | Azelastine HCL | Ocular diseases | Successfully deliver a hydrophobic medication in to the eye's anterior portion and cure allergic conjunctivitis | [66] |
| 17 | Sirolimus (SIR) | Cutaneous manifestation | It shows good stability | [67] |
| 18 | Methoxy poly (ethylene glycol)-polylactide copolymer | Metastasis breast cancers | This formulation shown tumour specific retaining and potent anti-cancer effectiveness against mouse breast cancer | [68] |
| 19 | Naproxen | Anti -inflammatory | The formulation shows good permeability across the skin | [69] |
| 20 | Clindamycin and isotretinoin | Acne vulgaris | The developed formulation is extremely effective against acne | [70] |
| 21 | Itraconazole | Fungal and keratitis | The created formulation shows long-lasting action without irritation, good permeability, and corneal toxicity | [71] |
| 22 | Tranylcypromine | Depression | Studies on drug penetration show that the medication's effect was extended release | [72] |
| 23 | Ceftiour | To treat deep infections in the bovine foot | Ceftiofur's rate of release was slowed down by a drop in the release medium's temperature | [73] |
| 24 | Timolol maleate | For increases ocular bioavailability | According to an in vivo study, 0.5 % timolol maleate gel increases the ocular bioavailability in albino rabbits. | [74] |
| 25 | Rapamycin | To improve skin permeation of poorly soluble drug | It increasing rapamycin's bioavailability and permeability | [75] |
| 26 | Posaconazole | Fungal ocular infections | This micellar gel is a potentially effective drug delivery improve drug ocular penetration and bioavailability | [76] |
Table 2: Reseach Work Done on Micellar Gel in Various Areas
Future Perspectives
According to recent research work, micellar gel is used for topical application to cure the various skin diseases or ocular diseases. This dosage form is noninvasive in nature, avoiding first pass metabolism. The limited solubility of hydrophobic drugs makes it difficult for researchers to deliver them to the targeted site. The hydrophobic medication that needs to be applied topically is the main focus here. Micelles help the medication quickly disintegrate at the intended location. Drug administration to a specific place enables sustained drug release while micellar microparticles enhance the solubility and dissolution of poorly soluble medications. These benefits of micellar microparticles open up a lot of potential for the topical distribution of hydrophobic drugs in the future, with increased efficacy and lower production costs. Micellar gel is potentially effective drug delivery to improve drug penetration and bioavailability. The present study of micellar gel with different types of drugs includes the various uses like breast cancer and ovarian cancer, onychomycosis, psoriasis, deep fungal infections, anti-inflammatory, anticancer, acne vulgaris, etc.
Acknowledgements:
The authors are thankful to the management of progressive education society for providing the necessary facilities to carry out the literature survey and related work.
Conflict of interests:
The authors declared that there is no conflict of interests.
References
- Abdulqader AA, Ahmed OH, Abduljabbar HH. Review of polymeric nanomicelles. J Pharm Negat Results 2022:853-6.
- Shiraishi K, Yokoyama M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci Technol Adv Mater 2019;20(1):324-36.
[Crossref] [Google Scholar] [PubMed]
- Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release 2005;109(1-3):169-88.
[Crossref] [Google Scholar] [PubMed]
- Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 2007;65(3):259-69.
[Crossref] [Google Scholar] [PubMed]
- Singh VK, Singh PK, Sharma PK, Srivastava PK, Mishra A. Formulation and evaluation of topical gel of acelofenac containing piparine. Indo Am J Pharm Res. 2013;3(7):5268-78.
- Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol Today 2000;3(6):198-204.
[Crossref] [Google Scholar] [PubMed]
- Toche VR, Deshmukh AS, Rabade AJ. Topical gels as drug delivery system-a comprehensive review. Int J Anal Exp Modal Anal 2021;13(5):664-81.
- Brassinne J, Mugemana C, Guillet P, Bertrand O, Auhl D, Bailly C, et al. Tuning micellar morphology and rheological behaviour of metallo-supramolecular micellar gels. J Soft Matter 2012;8(16):4499-506.
- Tamate R, Hashimoto K, Horii T, Hirasawa M, Li X, Shibayama M, et al. Self‐healing micellar ion gels based on multiple hydrogen bonding. Adv Mater 2018;30(36):1802792.
[Crossref] [Google Scholar] [PubMed]
- Moeinzadeh S, Jabbari E. Gelation characteristics, physico-mechanical properties and degradation kinetics of micellar hydrogels. Eur Polym J 2015;72:566-76.
[Crossref] [Google Scholar] [PubMed]
- Kamada M, Pierlot C, Molinier V, Aubry JM, Aramaki K. Rheological properties of wormlike micellar gels formed by novel bio-based isosorbide surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Colloids Surf. A: Physicochem. Eng. Asp 2018;536:82-7.
- Mugemana C, Joset A, Guillet P, Appavou MS, De Souza N, Fustin CA, et al. Structure of metallo‐supramolecular micellar gels. Macromol Chem Phys 2013;214(15):1699-709.
[Crossref] [Google Scholar] [PubMed]
- Ahmad Z, Shah A, Siddiq M, Kraatz HB. Polymeric micelles as drug delivery vehicles. RSC Adv 2014;4(33):17028-38.
- Bansal MO, Jamil SH. Micellar microparticles: A novel approach to topical drug delivery system. Int J Appl Pharm 2018;10:1-5.
- Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12(36):4669-84.
[Crossref] [Google Scholar] [PubMed]
- Ghezzi M, Pescina S, Padula C, Santi P, del Favero E, Cantù L, et al. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release 2021;332:312-36.
[Crossref] [Google Scholar] [PubMed]
- un Nabi SA, Sheraz MA, Ahmed S, Mustaan N, Ahmad I. Pharmaceutical gels: A review. RADS J Pharm Pharm Sci 2016;4(1):40-8.
- Sing SP, Tripathi DM. A comprehensive review on gel as transdermal drug delivery. Int J Pharm Pharm Sci 2021;23(1):70-82.
- Tuncaboylu DC, Sari M, Oppermann W, Okay O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011;44(12):4997-5005.
- Can V, Kochovski Z, Reiter V, Severin N, Siebenbürger M, Kent B, et al. Nanostructural evolution and self-healing mechanism of micellar hydrogels. Macromolecules 2016;49(6):2281-7.
- Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz Á. Anatomy and Function of the Skin. InNanoscience in dermatology. 2016. p. 1-14.
- Singh Malik D, Mital N, Kaur G. Topical drug delivery systems: A patent review. Expert Opin Ther Pat 2016;26(2):213-28.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Jin T, Zhuo RX. Methotrexate-loaded biodegradable polymeric micelles: Preparation, physicochemical properties and in vitro drug release. Colloids Surf B Biointerfaces 2005;44(2-3):104-9.
[Crossref] [Google Scholar] [PubMed]
- La SB, Okano T, Kataoka K. Preparation and characterization of the micelle‐forming polymeric drug indomethacin-incorporated poly (ethylene oxide)-poly (β‐benzyl L-aspartate) block copolymer micelles. J Pharm Sci 1996;85(1):85-90.
[Crossref] [Google Scholar] [PubMed]
- Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine 2010;6(6):714-29.
[Crossref] [Google Scholar] [PubMed]
- Kapare HS, Metkar SR. Micellar drug delivery system: A review. Pharm Reson 2020;2(2):21-6.
- Ai X, Zhong L, Niu H, He Z. Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles. Asian J Pharm sci 2014;9(5):244-50.
- Jiao Z, Liu N, Chen Z. Selection suitable solvents to prepare paclitaxel-loaded micelles by solvent evaporation method. Pharm Dev Technol 2012;17(2):164-9.
[Crossref] [Google Scholar] [PubMed]
- Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv 2006;3(1):139-62.
[Crossref] [Google Scholar] [PubMed]
- Rathod HJ, Mehta DP. A review on pharmaceutical gel. Int J Pharm Sci 2015;1(1):33-47.
- Soni A, Chaudhary A, Singla S, Goyal S. Review on: Novel Approach in Pharmaceutical Gel. J Curr Pharm Res 2018;9(1):2576-88.
- Bash BN, Prakasam K. Formulation and evaluations of gel containing fluconazole-antifungal agent. Int J Drug Dev Res 2011;3(4):20.
- Gulrez SK, Al-Assaf S, Phillips GO. Hydrogels: Methods of preparation, characterisation and applications. Prog Biophys Mol Biol 2011;118-50.
- Zhou W, Li C, Wang Z, Zhang W, Liu J. Factors affecting the stability of drug-loaded polymeric micelles and strategies for improvement. J Nanoparticle Res 2016;18:1-8.
- Abdeltawab H, Svirskis D, Sharma M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin Drug Deliv 2020;17(4):495-509.
[Crossref] [Google Scholar] [PubMed]
- Nasongkla N, Tuchinda P, Munyoo B, Eawsakul K. Preparation and characterization of MUC‐30‐loaded polymeric micelles against MCF‐7 cell lines using molecular docking methods and in vitro study. Evid Based Complement Alternat Med 2021;12(2):169-75.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, Chen H, Lan J, Song K, Wu X. Preparation and in vitro/in vivo evaluations of novel ocular micelle formulations of hesperetin with glycyrrhizin as a nanocarrier. Exp Eye Res 2021;202:108313.
[Crossref] [Google Scholar] [PubMed]
- Rupali S, Anupama D, Satish S, Shekhar S, Amisha V. Development and evaluation of niosomal gel for transdermalapplication of steroidal API. Int Res J Adv Sci Hub 2020;2(8):1-8.
- Sagar SV, Manogna K, Jyothi B. A Perspective overview on topical herbal gels. Res J Pharm Biol Chem Sci 2020; 11(6):113-9.
- Dou J, Zhang H, Liu X, Zhang M, Zhai G. Preparation and evaluation in vitroand in vivo of docetaxel loaded mixed micelles for oral administration. Colloids Surf B Biointerfaces 2014;114:20-7.
[Crossref] [Google Scholar] [PubMed]
- Gill KK, Nazzal S, Kaddoumi A. Paclitaxel loaded PEG5000–DSPE micelles as pulmonary delivery platform: formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur J Pharm Biopharm 2011;79(2):276-84.
[Crossref] [Google Scholar] [PubMed]
- Yu Y, Chen D, Li Y, Yang W, Tu J, Shen Y. Improving the topical ocular pharmacokinetics of lyophilized cyclosporine A-loaded micelles: Formulation, in vitroand in vivo studies. Drug Deliv 2018;25(1):888-99.
[Crossref] [Google Scholar] [PubMed]
- Manasa B, Shanmugam V, Prakash P. Formulation and evaluation of itraconazole proniosomal gel for topical drug delivery. Acta Sci Pharm Sci 2022;6:18-43.
- Kumar A, Dua JS. Formulation and evaluation of itraconazole niosomal gel. Asian J Pharm Res Dev 2018;6(5):76-80.
- Shilakari Asthana G, Asthana A, Singh D, Sharma PK. Etodolac containing topical niosomal gel: Formulation development and evaluation. J Drug Deliv 2016;4(5):1-8.
[Crossref] [Google Scholar] [PubMed]
- Prathyusha J, Yamani NS, Santhosh G, Aravind A, Naresh B. Formulation and evaluation of polyherbal face scrubber for oily skin in gel form. Int J Pharm Sci Drug Res 2019;11(4):126-8.
- Lapteva M, Mignot M, Mondon K, Möller M, Gurny R, Kalia YN. Self-assembled mPEG-hexPLA polymeric nanocarriers for the targeted cutaneous delivery of imiquimod. Eur J Pharm Biopharm 2019;142:553-62.
[Crossref] [Google Scholar] [PubMed]
- Giri MA, Bhalke RD. Formulation and evaluation of topical anti-inflammatory herbal gel. Asian J Pharm Clin Res 2019;12(7):252-5.
- Suksiriworapong J, Rungvimolsin T, A-gomol A, Junyaprasert VB, Chantasart D. Development and characterization of lyophilized diazepam-loaded polymeric micelles. AAPS PharmSciTech 2014;15:52-64.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Ouyang WQ, Wei YP, Syed SF, Hao CS, Wang BZ, et al. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int J Nanomedicine 2016:5971-87.
[Crossref] [Google Scholar] [PubMed]
- Patra A, Satpathy S, Shenoy AK, Bush JA, Kazi M, Hussain MD. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int J Nanomedicine 2018:2869-81.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Wang J, Zhang X, Lu W, Zhang Q. A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J Control Release 2009;135(2):175-82.
[Crossref] [Google Scholar] [PubMed]
- Krawczyk-Santos AP, Marreto RN, Concheiro A, Alvarez-Lorenzo C, Taveira SF. Poly (pseudo) rotaxanes formed by mixed micelles and α-cyclodextrin enhance terbinafine nail permeation to deeper layers. Int J Pharm X 2022;4:100118.
[Crossref] [Google Scholar] [PubMed]
- Khurana B, Arora D, Narang RK. QbD based exploration of resveratrol loaded polymeric micelles based carbomer gel for topical treatment of plaque psoriasis: in vitro, ex vivo and in vivo studies. J Drug Deliv Sci Technol 2020; 59:1-15.
- Xu H, Wen Y, Chen S, Zhu L, Feng R, Song Z. Paclitaxel skin delivery by micelles-embedded Carbopol 940 hydrogel for local therapy of melanoma. Int J Pharm 2020;587:1-11.
[Crossref] [Google Scholar] [PubMed]
- Duan Y, Cai X, Du H, Zhai G. Novel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcumin. Colloids Surf B Biointerfaces 2015; 128:322-30.
[Crossref] [Google Scholar] [PubMed]
- Laurano R, Boffito M. Thermosensitive micellar hydrogels as vehicles to deliver drugs with different wettability. Front Bioeng Biotechnol 2020;8(1):708.
[Crossref] [Google Scholar] [PubMed]
- Varshosaz J, Taymouri S, Minaiyan M, Rastegarnasab F, Baradaran A. Development and in vitro/in vivo evaluation of HPMC/chitosan gel containing simvastatin loaded self-assembled nanomicelles as a potent wound healing agent. Drug Dev Ind Pharm 2018;44(2):276-88.
[Crossref] [Google Scholar] [PubMed]
- Sai N, Dong X, Huang P, You L, Yang C, Liu Y, et al. A novel gel-forming solution based on PEG-DSPE/Solutol HS 15 mixed micelles and gellan gum for ophthalmic delivery of curcumin. Molecules 2019;25(1):81.
[Crossref] [Google Scholar] [PubMed]
- Sharma PK, Bhatia SR. Effect of anti-inflammatories on Pluronic® F127: Micellar assembly, gelation and partitioning. Int J Pharm 2004;278(2):361-77.
[Crossref] [Google Scholar] [PubMed]
- Chen D, Yu H, Sun K, Liu W, Wang H. Dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery. Drug Deliv 2014;21(4):258-64.
[Crossref] [Google Scholar] [PubMed]
- Vigyan S, Manish K, Kamla P. Development of miconazole nitrate loaded micellar gel for improved topical delivery. Thai J Pharm Sci 2016;15(2):87-94.
- Pokharkar V, Suryawanshi S, Dhapte-Pawar V. Exploring micellar-based polymeric systems for effective nose-to-brain drug delivery as potential neurotherapeutics. Drug Deliv Transl Res 2020;10:1019-31.
[Crossref] [Google Scholar] [PubMed]
- Figueroa-Ochoa EB, Villar-Alvarez EM, Cambón A, Mistry D, Llovo J, Attwood D, Barbosa S, Soltero JA, Taboada P. Lenghty reverse poly (butylene oxide)-poly (ethylene oxide)-poly (butylene oxide) polymeric micelles and gels for sustained release of antifungal drugs. Int J Pharm 2016;510(1):17-29.
[Crossref] [Google Scholar] [PubMed]
- Huang P, Zhang Y, Wang W, Zhou J, Sun Y, Liu J, et al. Co-delivery of doxorubicin and 131I by thermosensitive micellar-hydrogel for enhanced in situ synergetic chemoradiotherapy. J Control Release 2015;220:456-64.
[Crossref] [Google Scholar] [PubMed]
- Mansour M, Mansour S, Mortada ND, Abd ElHady SS. Ocular poloxamer-based ciprofloxacin hydrochloride in situ forming gels. Drug Dev Ind Pharm 2008;34(7):744-52.
[Crossref] [Google Scholar] [PubMed]
- Devi S, Saini V, Kumar M, Bhatt S, Gupta S, Deep A. A novel approach of drug localization through development of polymeric micellar system containing azelastine HCl for ocular delivery. Pharm Nanotechnol 2019;7(4):314-27.
[Crossref] [Google Scholar] [PubMed]
- Quartier J, Lapteva M, Boulaguiem Y, Guerrier S, Kalia YN. Polymeric micelle formulations for the cutaneous delivery of sirolimus: A new approach for the treatment of facial angiofibromas in tuberous sclerosis complex. Int J Pharm 2021;604:120736.
[Crossref] [Google Scholar] [PubMed]
- Luo L, Zhang Q, Luo Y, He Z, Tian X, Battaglia G. Thermosensitive nanocomposite gel for intra-tumoral two-photon photodynamic therapy. J Control Release 2019;298:99-109.
[Crossref] [Google Scholar] [PubMed]
- Puig-Rigall J, Blanco-Prieto MJ, Aydillo C, Radulescu A, Molero-Vilchez D, Dreiss CA, et al. Poloxamine/D-α-tocopheryl Polyethylene Glycol Succinate (TPGS) mixed micelles and gels: Morphology, loading capacity and skin drug permeability. J Mol Liq 2021;324:1-10.
- Sharma G, Yachha Y, Thakur K, Mahajan A, Kaur G, Singh B, et al. Co-delivery of isotretinoin and clindamycin by phospholipid-based mixed micellar system confers synergistic effect for treatment of acne vulgaris. Expert Opin Drug Deliv 2021;18(9):1291-308.
- Munmun Jaiswal, Manish Kumar, Kamla Pathak. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf B Biointerfaces 2015;12(2):13023-30.
[Crossref] [Google Scholar] [PubMed]
- Chaudhari SP, Shinde PU. Formulation and characterization of tranylcypromine loaded polymeric micellar in-situ nasal gel for treatment of depression. Technology 2020;5(4):149-65.
- Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release 2002;85(1-3):73-81.
[Crossref] [Google Scholar] [PubMed]
- El-Kamel AH. in vitro and in vivoevaluation of pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm 2002;241(1):47-55.
[Crossref] [Google Scholar] [PubMed]
- le Guyader G, Do B, Rietveld IB, Coric P, Bouaziz S, Guigner JM, et al. Mixed polymeric micelles for rapamycin skin delivery. Pharmaceutics 2022;14(3):569.
[Crossref] [Google Scholar] [PubMed]
- Durgun ME, Mesut B, Hacioglu M, Gungor S, Ozsoy Y. Optimization of the micellar-based in situ gelling systems posaconazole with quality by design (QbD) approach and characterization by in vitro studies. Pharmaceutics 2022;14(3):526.
[Crossref] [Google Scholar] [PubMed]