- *Corresponding Author:
- Ayon Bhattacharya
Department of Pharmacology
KPC Medical College
WBUHS, Kolkata-700 032, India
E-mail: ayon.bhattacharya23@gmail.com
| Date of Received | 10 August 2020 |
| Date of Revision | 13 December 2020 |
| Date of Acceptance | 23 December 2020 |
| Indian J Pharm Sci 2020;82(6):996-1005 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study is to determine the neuropharmacological activities of the methanol extract of Aloe vera leaf and identify the potential bioactive compounds present in the same responsible for the activities through Gas chromatography–mass spectrometry analysis. The study is done on Wistar albino rats and composed of 5 groups, methanol extract of Aloe vera leaf at 200, 400, 800 mg/kg, normal saline (control) and standard drugs Phenytoin and Diazepam. Antiepileptic activities are done using maximal electroshock and pentylenetetrazole induced convulsion models and anxiolytic sedative activities using the staircase test, actophotometer test and sleep prolongation test. The chemometric analysis is performed using Clarus 500 Perkin Gas Chromatograph coupled with a mass detector, Turbo mass gold–Perkin Elmer Turbomass 5.1 spectrometer with an Elite-1 (100 % dimethyl polysiloxane), 30 m×0.25 mm ID×0.25 μm of the capillary column. The results demonstrated anxiolytic potential at 200 and 400 mg/kg and sedative activity at 800 mg/kg comparable to Diazepam. The methanolic extract of Aloe vera leaf at 800 mg/kg displayed antiepileptic activity compared to Phenytoin. The Gas chromatography–mass spectrometry analysis confirmed the presence of phytol (23.76), alpha-tocopherol (4.67), maltol (3.69), humulene (6.58), caryophyllene (2.73), n-hexadecanoic acid (2.16), hexadecanoic acid methyl ester (3.70), squalene (3.51), 9,12,15-octadecatrienoic acid methyl ester (2.12), which are antioxidant and anti-inflammatory compounds. These plant derived bioactive compounds are demonstrated to have antianxiety, antiepileptic, sedative hypnotic and neuroprotective potential in neurodegenerative models and might serve as future prospective drug candidates in neurodegenerative disorders).
Keywords
Epilepsy, anxiety, oxidative stress, neuroinflammation, neurodegeneration, sedative, hypnotic
Stressful situations in life induce metabolic disturbances, contributing to oxidative damage in cells and tissues, consequently leading to chronic neurological disorders[1]. Anxiety, depression and epilepsy are the most common progressive neurological disorders causing cognitive impairments in millions of people[2-4]. The long term impact of oxidative stress is neurodegeneration, neuroinflammation and direct neuronal damage seen in neurological disorders[5]. The future therapeutic strategies in neurological disorders are likely to incorporate a regimen of antioxidant and anti-inflammatory compounds that are neuroprotective, preventing disease progression and several molecular pathways leading to neuronal injury.
Aloe vera is a plant of immense ethnopharmacological importance, rich traditional history and several pharmacological uses. The perennial, succulent, drought resistant and hardy nature of the plant makes it household friendly and an excellent option for agroforestry[6]. The leaf extract of Aloe vera is known for wound healing, antifungal, antidiabetic, immunomodulator and gastroprotective properties[7]. Moreover, several studies proved that Aloe vera has neuroprotective potential and crosses the blood brain barrier[8,9]. Aloe vera extract restored mitochondrial damage in rat brain and pheochromocytoma cells[8]. Pretreatment with Aloe vera attenuated neuronal damage in rats induced with spinal cord ischemia reperfusion injury[9]. In a subsequent study, 8 w treatment with Aloe vera gel alleviated the diabetes induced deficits such as increased anxiety/ depression-like behavior, reduced locomotor activities, decreased memory performance and increased stress related behaviors suggesting an interaction between hypoglycemic and antioxidative properties of Aloe vera[10]. Ultraviolet visible-spectrophotometer analyses revealed flavonoids, tannins and phenolic content in the Aloe vera extract, which are found to possess neuroprotective properties[11-13]. The aqueous extract of Aloe vera exhibited significant anticonvulsant and sedative hypnotic activities in the rodent model[14,15]. However, an extensive literature search suggests that the antiepileptic, anxiolytic and sedative activity of methanolic extract of the same has not been studied so far. In the present study, we chose methanol solvent because of its amphiphilic nature and its ability to detects minute traces of bioactive constituents[16]. Methanolic plant extract produces the highest yield of phenolics, flavonoids, alkaloids and terpenoids, suggesting methanol to be the ideal solvent for isolating highly polar bioactive compounds[17]. Soxhlation is used as this method of extraction as it provides a maximum yield of phytochemicals compared to other extraction methods[18]. Furthermore, methanol extracts polar metabolites of compounds with both medium and low polarity by the co-solubilization process, penetrate the cell membrane of plant and extracts a high amount of intracellular phytoconstituents[19].
We conducted a pilot study at our lab in rats using intraperitoneal (ip) administration of methanolic extract of Aloe vera (MEAV) leaf at 100, 200, 400, and 800 mg/kg[15-20]. No pharmacological effect in rats is noted at 100 mg/kg. The animals on observation post administration of 200, 400, and 800 mg/kg for 1 w revealed no mortality or toxicity signs. However, we observed increased defecation frequency in 400 and 800 mg/kg that normalized after 3 d[16,21]. The dose of Phenytoin and Diazepam are selected based on previous literature where the former is used as a standard antiepileptic drug[22] and the biphasic profile of the latter, i.e., antianxiety at 1 mg/kg and sedative at 2 mg/kg[15,23]. Henceforth, in the present study, we employed five different models to evaluate the antiepileptic, anxiolytic and sedative activity of MEAV and identified the compounds present in the extract through Gas chromatography–mass spectrometry (GC-MS) analysis. An extensive literature search in Google Scholar, PubMed, Web of Science and EBSCO, revealed antianxiety, antiepileptic, sedative hypnotic and neuroprotective properties of the bioactive compounds revealed in GC-MS, which we have summarized in the discussion section.
Materials and Methods
Phenytoin purchased from Sigma Aldrich Co. (St. Louis, MO, USA), pentylenetetrazole (Sigma-Aldrich, USA), diazepam (Calmpose injection, Ranbaxy Laboratories Ltd, New Delhi, India) and methanol (analytical grade) from Simalin Chemical Industries Private Limited, Vadodara, India.
Healthy Wistar strain of albino rats (8 w old) of either sex, weighing between 150-250 g, is selected. According to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and good laboratory practice (GLP) guidelines, all experiments are conducted. Animals that could not be rehabilitated are painlessly euthanized using an anesthetic agent (ketamine HCl), according to the CPCSEA. The Institutional Animal Ethical Committee (No. 003/IAEC/IMS & SH/ SOAU) approved the study.
Collection of plant and preparation of plant extract:
The leaves of Aloe vera are obtained from Bhubaneswar, India, during the day hours of the summer season (May), 2016 and verified from the Department of Taxonomy and Conservation, Regional Plant Resource Centre, Bhubaneswar, India[24]. A voucher specimen is submitted (SPS/SOAU-23) to the herbarium, Department of Pharmacognosy. The air dried Aloe vera leaves are first ground and then extracted using methanol in the Soxhlet apparatus for 24 h. The filtered supernatant is then concentrated and dried using a rotary evaporator at reduced pressure at 40°. The yield generated from the powdered extract of Aloe vera leaves is 59.92 % (59.92 g of extract out of 100 g leaves) relative to the weight of the leaves used. The filtrate is lyophilized in freeze dried form and stored in -20°. We prepared the i.p formulation by weighing the required amount of extract and suspended it in a physiological saline solution containing 1 % Tween-80 (v/v) on the day of the experiment[25-27].
Grouping:
The study design used was a randomized control experimental study. Wistar rats of either sex are randomized and assigned to five groups, each group of six animals (n=6). The individual group animals received ip injection; Group 1: standard drug or positive control group-Phenytoin (30 mg/kg)/diazepam (1 mg/kg/2 mg/kg), Group 2: negative control- 0.9 % normal saline (10 ml/kg), Group 3, 4, 5:200, 400 and 800 mg/kg of MEAV, respectively. The detailed toxicological evaluation of methanolic extract of Aloe vera in rats is already studied, which did not produce any toxicity during acute and subacute treatment[20].
Evaluation of antiepileptic activity:
The antiepileptic activity was evaluated using two methods, the maximal electroshock induced seizures (MES) and the pentylenetetrazole (PTZ) induced seizures. MES was performed according to Giardina and Gassier[28]. Percent inhibition of seizures was calculated with the formula[29], Percentage inhibition of seizure=100(1-(DT/DC), where DT is the duration of seizure after test drug treatment, DC is the duration of seizure without any drug treatment. PTZ induced seizure model was established according to the procedure listed in vogel[30].
Actophotometer test and staircase test:
It consists of 6 inbuilt light sources, a photosensor and a digital counter for recording the locomotor activity[31]. Percentage reduction of activity score is calculated using the formula, Percentage reduction in motor activity=(Wa–Wb/Wa)×100 %, where Wa is the basal score and Wb is the score after treatment[32]. The staircase test for evaluating anxiolytic activity was performed according to the method described by Thiebot et al. (1973)[33].
Prolongation of Sleep induction test:
MEAV (200, 400 and 800 mg/kg) and diazepam (2 mg/kg) are administered ip 30 min before the administration of 40 mg/kg i.p. thiopentone sodium[34]. The time from the onset of sleep (loss of righting reflex) to recovery time is recorded in min.
GC-MS Instrument and Analysis:
The analysis is performed according to the described method[35]. The National Institute of Standards and Technology (NIST 11) library is used in this study to match the reference mass spectra of compounds with the mass spectral pattern of chemical constituents. The unit of expression of individual compounds in this study is in percent peak areas compared to the total peak area.
Statistical Analysis:
The G*Power 3.1.9.2 software is used to calculate the sample size. For one-way analysis of variance (ANOVA), effect size (f) is taken as 0.70, α error probability (0.05) and power (0.80). The normality assumption is satisfied in the actophotometer activity score, onset of sleep and recovery, number of steps (climbs and rears), mean clonic, tonic, onset, hind limb extension (HLE) and hind limb flexion (HLF). Thus, parametric test ANOVA is done to compare the treatment means followed by post-hoc Bonferroni test for pairwise comparison of treatment means. Comparison of mean stupor, recovery and total duration in MES test is done using the nonparametric Kruskal-Wallis test and pairwise comparison is done using the Mann-Whitney U test, as they did not satisfy normality distribution. The significance level of p<0.05 is considered.
Results and Discussion
The statistical test ANOVA showed significant differences among the treatment groups in the various parameters of the MES model (Table 1). ANOVA followed by Bonferroni’s multiple pairwise comparison test showed significant differences among the group comparisons, except for comparisons between MEAV 800 mg/kg and Phenytoin 30 mg/kg [p=1.00] in the onset of seizures and the HLF. In the HLE parameter, no significant difference is found in comparing MEAV 200 with 400 mg/kg [p=1.00]; MEAV 200 with 800 mg/ kg [p=1.00]; MEAV 400 with 800 mg/kg [p=1.00] and between MEAV 800 mg/kg and Phenytoin 30 mg/kg [p=1.00]. Kruskal Wallis test showing the mean ranks in the stupor, recovery and total duration is displayed and significant differences among the treatment groups [p=0.00] are noted (Table 2). Furthermore, Mann Whitney’s U test showed significant [p<0.05] differences in the stupor and recovery among all group comparisons except MEAV 200 with 400 mg/kg [p=0.37] and MEAV 400 with 800 mg/kg [p=0.19]. In the total duration parameter, significant differences in [p<0.05] all the pairwise comparisons are noted.
| Treatment | Onset (s) | Duration of HLE (s) | Duration of HLF (s) | Number of animals survived/used | % inhibition of seizure |
|---|---|---|---|---|---|
| Phenytoin (30 mg/kg) | 6.90±0.49 | 4±1.79 | 7.5±1.87 | 6/6 | 87.13 |
| Saline (10 ml/kg) | 0.94±0.13 | 32.17±7.41 | 46±3.90 | 3/6 | |
| MEAV (200 mg/kg) | 2.87±0.21 | 9.33±1.75 | 18.83±1.47 | 6/6 | 69.46 |
| MEAV (400 mg/kg) | 3.82±0.33 | 8.17±1.60 | 16.83±1.94 | 6/6 | 72.66 |
| MEAV (800 mg/kg) | 6.92±0.60 | 6.50±1.05 | 6.17±2.32 | 6/6 | 77.58 |
| p value | 0.00 | 0.00 | 0.00 |
Values expressed as mean±SD (n=6). Statistical test: ANOVA, Level of significance: p<0.05; Significant difference among the treatment groups noted. MEAV: methanol extract of Aloe vera; MES: maximal electroshock seizure, HLE: hind limb extension; HLF: hind limb flexion; Administration: intraperitoneal (all groups)
Table 1: Comparison of Parameters in the MES Model among Treatment Groups
| Treatments (n=6) |
Stupor and recovery (s) | Total duration (s) | ||
|---|---|---|---|---|
| Mean | Mean Rank | Mean | Mean Rank | |
| Phenytoin 30 mg/kg | 41±4.94 | 3.50 | 52.33±2.25 | 3.50 |
| Saline (10 ml/Kg) | 328.50±183.47 | 27.50 | 406.67±186.65 | 27.50 |
| MEAV 200 mg/Kg | 96±6.07 | 19.42 | 124.17±5.08 | 20.75 |
| MEAV 400 mg/Kg | 86.17±15.30 | 15.92 | 111.17±12.94 | 15.75 |
| MEAV 800 mg/Kg | 78.50±5.65 | 11.17 | 91.17±4.40 | 10 |
| Kruskall wallis p value | 0.00 | 0.00 | ||
Values expressed as mean±SD (n=6) and ranks. MES: maximal electroshock seizure. Statistical test: Kruskal Wallis. Level of significance p<0.05; Significant difference among the ranks in the treatment groups are seen. MEAV: methanol extract of Aloe vera. Administration: intraperitoneal (all groups).
Table 2: Comparison of Stupor, Recovery and Total Duration among Treatments in MES Model
ANOVA showed significant differences in the clonic and tonic phase [p=0.00] (Table 3). Thereafter, multiple pairwise comparisons (Bonferroni’s test) revealed significant [p<0.05] differences in the onset of the clonic phase in all treatment groups. The onset of the tonic phase showed no significant difference between MEAV 800 mg/kg and Phenytoin 30 mg/kg [p=1.00].
| Treatment | The onset of Clonic (s) | The onset of tonic (s) | Number of animals survived/used |
|---|---|---|---|
| Phenytoin (30 mg/kg) | 0.26±0.04 | 0.15±0.03 | 6/6 |
| Saline (10 ml/kg) | 40±2.83 | 60±1.41 | 2/6 |
| MEAV (200 mg/kg) | 29.75±2.22 | 70±1.83 | 4/6 |
| MEAV (400 mg/kg) | 72±3.67 | 102.20±5.36 | 5/6 |
| MEAV (800 mg/kg) | 201.67±5.35 | 0.21±0.02 | 6/6 |
| p value | 0.00 | 0.00 |
Values are expressed in mean±SD (n=6). PTZ: pentylenetetrazole. Statistical test: ANOVA. Level of significance p<0.05; Significant difference among the treatment groups noted. MEAV: methanol extract of Aloe vera. Administration: intraperitoneal (all groups)
Table 3: Comparison of Parameters in PTZ-Induced Epilepsy among the Treatment Groups
On analyzing the mean number of steps climbed and rears using ANOVA, significant differences among the treatment groups [p=0.00] is seen (Table 4). In the number of steps climbed, post hoc Bonferroni’s test showed significant differences [p<0.05] among the comparisons in all the treatment groups except for Diazepam and MEAV 200 mg/kg [p=0.12] and between MEAV 200 and MEAV 400 mg/kg [p=1.00]. In the number of rears, no significant difference is observed among the following comparisons: Diazepam and MEAV 400 mg/kg [p=0.77]; Diazepam and MEAV 800 mg/kg [p=1.00]; normal saline and MEAV 200 mg/ kg [p=0.48]; MEAV 200 mg/kg and MEAV 400 mg/ kg [p=0.77]. In the actophotometer test, an ANOVA test is done to compare the treatment groups before and after drug administration at 300 and 600 sec (Table 5). No significant difference is noted in the activity score at the time points 300 [p=0.61] and 600 sec [p=0.78] before drug administration. However, following drug administration, the activity score at 300 [p=0.00] and 600 sec [p=0.00] showed a significant difference. Bonferroni’s test showed no significant difference [p=0.14] between the mean score of positive control (Diazepam) and MEAV 800 mg/kg treatment group for 300 sec but significantly differed [p=0.00] between MEAV 800 mg/kg and positive control (Diazepam) for 600 sec after drug administration. The percentage inhibition of activity scores following MEAV administration at 300 sec is calculated using the formula as mentioned above and revealed 82.6 % for Diazepam and 55.33 %, 64.45 %, 81.11 % for MEAV 200, 400 and 800 mg/kg, respectively. Similarly, the percentage inhibition of activity scores for 600 sec showed 84.15 % for Diazepam, 59.02 %, 70.68 %, 78.07 % for MEAV 200, 400 and 800 mg/kg respectively.
| Treatment | Number of steps climbed | Number of rears |
|---|---|---|
| Diazepam (1 mg/kg) | 12.67±1.21 | 2.33±0.81 |
| Saline (10 ml/kg) | 6.17±1.47 | 10.67±2.42 |
| MEAV (200 mg/kg) | 10.83±1.16 | 7.67±4.13 |
| MEAV (400 mg/kg) | 10.17±1.16 | 5±2.68 |
| MEAV (800 mg/kg) | 3.17±0.75 | 0.83±0.75 |
| p value | 0.00 | 0.00 |
Values are expressed in mean±SD (n=6). Statistical test, ANOVA. Level of significance p<0.05; Significant difference among the treatment groups observed. MEAV: methanol extract of Aloe vera. Administration: intraperitoneal (all groups)
Table 4: Comparison of Parameters among Treatment Groups in the Staircase Test
| Treatment | Activity score before drug administration (300 s) | Activity score before drug administration (600 s) | Activity score after drug administration (300 s) | Activity score after drug administration (600 s) |
|---|---|---|---|---|
| Diazepam (1 mg/kg) | 221.33±4.32 | 311.33±4.32 | 38.50±2.17 | 49.33±4.32 |
| Saline (10 ml/kg) | 219.50±3.27 | 309±2.68 | 229.50±2.51 | 263.67±3.93 |
| MEAV (200 mg/kg) | 220.17±2.79 | 309.17±2.93 | 98.83±2.94 | 126.67±3.27 |
| MEAV (400 mg/kg) | 220.83±2.48 | 309.83±4.07 | 78.50±1.64 | 90.83±1.47 |
| MEAV (800 mg/kg) | 222.33±3.08 | 310.17±2.93 | 42±2.09 | 68±2.09 |
| p value | 0.61 | 0.78 | 0.00 | 0.00 |
Values are expressed in mean±SD (n=6). Statistical test, ANOVA. Level of significance p<0.05; Significant difference among the treatment groups observed. MEAV: methanol extract of Aloe vera. Administration: intraperitoneal (all groups)
Table 5: Comparison of Activity Scores Before and after Drug Administration in Actophotometer Test among the Treatment Groups
In the sleep induction test, ANOVA showed significant differences [p=0.00] among the treatment groups in both the onset and recovery of sleep parameters (Table 6). In the onset of sleep parameter, multiple pairwise comparison using Bonferroni’s test revealed no significant difference between the following pairwise comparisons: negative control (normal saline) and MEAV 200 mg/kg [p=1.00]; MEAV 200 and 400 mg/kg [p=0.17]; and positive control (Diazepam 2mg/kg) and MEAV 800 [p=1.00]. In the recovery of sleep outcome, the pairwise comparison revealed a significant difference [p<0.05] in all group comparisons, except between the normal saline group and MEAV 200 mg/kg [p=1.00].
| Treatment | Onset of sleep (min) | Recovery from sleep (min) |
|---|---|---|
| Diazepam (2 mg/kg) | 4.79±0.58 | 217±2.63 |
| Saline (10 ml/kg) | 7.67±0.47 | 89.33±3.33 |
| MEAV (200 mg/kg) | 7.50±0.42 | 89.50±2.59 |
| MEAV (400 mg/kg) | 6.86±0.39 | 130.83±20.10 |
| MEAV (800 mg/kg) | 4.98±0.25 | 179.17±9.70 |
| p value | 0.00 | 0.00 |
Values are expressed in mean±SD (n=6). Statistical test, ANOVA. Level of significance p<0.05; Significant difference among the treatment groups observed. MEAV: methanol extract of Aloe vera. Administration: intraperitoneal (all groups)
Table 6: Effect of Methanol Leaf Extract of Aloe Vera on Sleep Induction Test in Rats
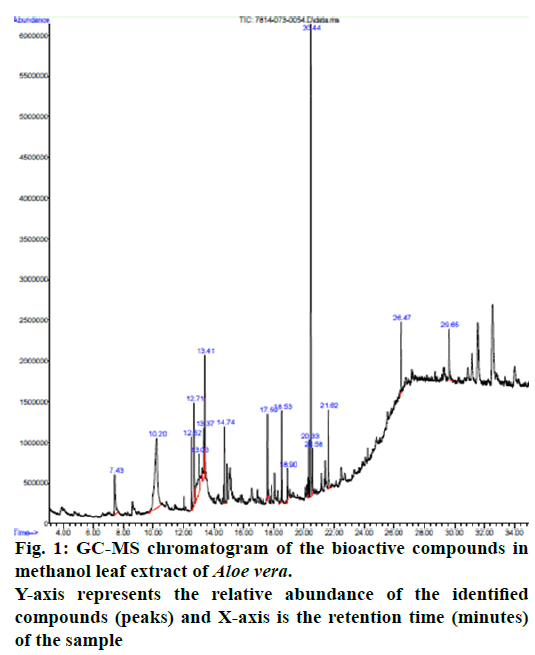
The GC-MS analysis of MEAV revealed 16 compounds. The GC-MS chromatogram, in its entirety, is shown in fig. 1. GC-MS analysis confirmed the presence of terpenoids (such as Alpha-tocopherol, Phytol, Humulene, Caryophyllene, Squalene), pyrans (Maltol), fatty acid (Hexadecanoic acid methyl ester, 9,12,15-Octadecatrienoic acid methyl ester and n-Hexadecanoic acid). Among the bioactive constituents, phytol is found to be at the highest level (23.76 %). The descriptions of the detected bioactive constituent are presented in (Table 7 & 8).
| Retention Time (min) | Name of the compound | Molecular Formula |
Molecular Weight (g/mol) |
Peak area (%) | Nature of the compound |
|---|---|---|---|---|---|
| 29.644 | Alpha-Tocopherol (Vitamin E) | C29H50O2 | 430.717 | 4.67 | Alcohol |
| 20.440 | Phytol | C20H40O | 296.539 | 23.76 | Diterpene |
| 13.026 | Humulene | C15H24 | 204.357 | 6.58 | Terpene |
| 12.707 | (E)-hex-3-enyl isobutyl carbonate | C11H20 O3 | 200.275 | 6.40 | Carbonic acid ester |
| 13.413 | (Z)-hex-3-enyl isobutyl carbonate | C11H20O3 |
200.278 | 4.98 | Carbonic acid ester |
| 7.433 | Maltol | C6H6O3 | 126.111 | 3.69 | Aromatic compound |
| 12.521 | Caryophyllene | C15H24 | 204.357 | 2.73 | Terpene |
| 18.538 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.4507 | 3.70 | Fatty acid ester |
| 21.621 | Naphthalene, 1-isocyano | C11H7N | 153.1800 | 4.61 | Polycylic aromatic hydrocarbon |
| 26.465 | Squalene | C30H50 | 410.73 | 3.51 | Triterpene |
| 20.581 | Methyl stearate | C19H38O2 | 298.511 | 1.88 | Esterified Fatty acid |
| 20.329 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z, Z, Z)- | C19H32O2 | 292.456 | 2.12 | Fatty acid (Linolenic acid) |
| 18.902 | n-Hexadecanoic acid | C16H32O2 | 256.43 | 2.16 | Saturated fatty acid |
| 17.588 | Bicyclo[3.1.1]heptane, 2,6,6-trimethyl- | C10H18 | 138.2499 | 3.04 | Monoterpene ketone |
| 13.368 | (+)-epi-Bicyclosesquiphellandrene | C15H24 | 204.357 | 2.59 | Hydrocarbon sesquiterpenes |
| 10.204 | 2-t-Butyl-5-hydroxymethyl-5-methyl-[1,3]dioxolan-4-one | C9H16O4 | 188.223 | 20.55 | Cyclic ether |
Table 7: Characteristics of Phytochemical Compounds Identified in Methanol Leaf Extract of Aloe Vera
| NAME OF COMPOUNDS | CHEMICAL STRUCTURE |
|---|---|
| Alpha-Tocopherol (Vitamin E) | 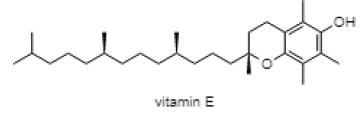 |
| Phytol | 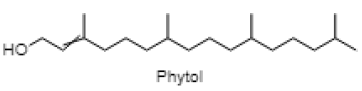 |
| Humulene | 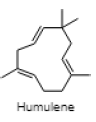 |
| Maltol | 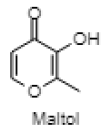 |
| Caryophyllene | 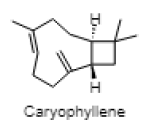 |
| Hexadecanoic acid, methyl ester | 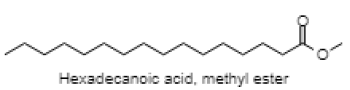 |
| Squalene | 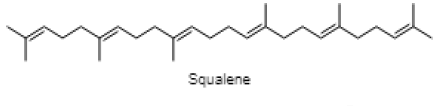 |
| n-Hexadecanoic acid | 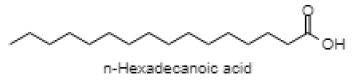 |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 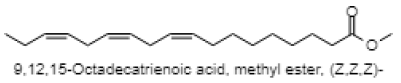 |
Table 8: Structures of Phytochemical Compounds Identified in Methanol Leaf Extract of Aloe Vera
In this study, the MEAV treatment groups showed a gradual decrease in the duration of MES-induced HLE comparable to Phenytoin. Furthermore, MEAV treated groups displayed an increase in the latency to the onset of clonic convulsion duration compared to Phenytoin in the PTZ induced epilepsy model, which corroborates with the findings reported by Rathor et al. (Table 3). However, Rathor and associates conducted both acute and chronic epileptic studies using aqueous extract of Aloe vera at 100, 200, and 400 mg/kg and reported significant antiepileptic activity at 200 and 400 mg/kg. Additionally, our study extended the findings from the Rathor group by demonstrating the gradual increase in the latency of seizure, reduced duration of HLF in comparison to control (Table 1). Moreover, MEAV at 800 mg/kg exhibited strong antiepileptic potential comparable to Phenytoin by almost abolishing the onset of the tonic phase (Table 3). The gradual inhibition of seizure with increasing doses of MEAV and no mortality rate in the MEAV treated groups suggested an antiepileptogenic potential of the methanolic extract against grand mal epilepsy and petit mal epilepsy[36].
In the staircase test, the reduction in the number of rears without suppressing locomotor activity[37] reflected the anxiolytic nature of MEAV. Diazepam and MEAV (200 and 400 mg/kg) treated groups showed an increase in the steps, which could be due to the increased exploratory behavior secondary to reduced anxiety. Moreover, a decrease in the number of steps and rears at 800 mg/ kg suggested the sedative ability of MEAV. The sleep prolongation test showed a decrease in the latency of sleep from MEAV 200 to 800 mg/kg and an increase in the recovery time in all the respective doses suggested a possible sedative effect[38]. However, in our study, the sedative activity of MEAV 200 and 400 mg/kg in the sleep induction test does not corroborate with actophotometer and staircase test suggesting that MEAV might be potentiating the effect of thiopentone sodium showing the Central nervous system (CNS) depressive effect of the drug, but may not be able to induce the sedative effect by itself in the test doses used. Our study suggested anxiolytic activity at low doses (200 and 400 mg/kg) and sedative activity at a higher dose (800mg/kg), reflecting a biphasic profile of Aloe vera, which aligns with previous studies[39-41]. However, the previous studies are conducted in different extracts of Aloe vera with different doses and experimental designs.
Oxidative stress is associated with altered reactive oxygen species (ROS), reactive nitrogen species (RNS) and nitric oxide (NO) signaling pathways[42,43]. Several studies have suggested the implication of oxidative stress in the etiology of epilepsy and seizure induced neurodegeneration[44,45]. Several animal studies have demonstrated the induction of oxidative stress caused anxiety like behavior in animals and treatment with antioxidants prevented anxiety behavior, suggesting a causal relationship between oxidative stress and anxiety disorders[46].
Our GC-MS result corroborates with the previous studies that evaluated the active constituents of ethanolic and hexane extract of Aloe vera that identified compounds same as in our study such as n-hexadecanoic acid, 9,12,15-Octadecatrienoic acid, methyl ester, (Z, Z, Z)-, squalene and hexadecanoic acid, methyl ester(35,36). However, the present study has detected additional antioxidant compounds such as phytol, vitamin E, maltol, humulene/caryophyllene, squalene and α-linolenic acid, which is reported to exhibit CNS activities, which is discussed below. Among these bioactive compounds, vitamin E and squalene are thermolabile in nature[47,48]. However, the thermoprofile data of other bioactive compounds were not found.
Phytol is reported in reducing pilocarpine induced seizures, inferring that the anticonvulsant mechanism does not involve a Gamma aminobutyric acid (GABA) ergic system and might be due to interaction with other receptors (serotonin, noradrenaline and glutaminergic)[49]. However, the anxiolytic action of phytol in animal models by the same authors a year later suggested that phytol might interact with GABA-A receptor subunits mediating benzodiazepine effects[50]. The anti-inflammatory effect of phytol is reported in the acute inflammation model, which might be due to the inhibition of cytokine production and oxidative stress[51]. The neuroprotective effect of alpha tocopherol (vitamin E) in oxidative stress associated with neurological disorders suggested the direct involvement of alpha tocopherol in neuronal survival by reducing free radical injury and oxidative stress[52]. Notably, post seizure vitamin E administration significantly reduced inflammation induced brain damage by lowering the lipid peroxidation levels in the hippocampus[53]. Maltol is also reported as a potential therapeutic candidate for the novel neuroprotective/regenerative therapeutic strategy in neurodegenerative disease related to oxidative stress[54]. Pretreatment with maltol resulted in attenuation of neuronal loss in hippocampus and restoration in the oxidative stress biomarkers to normal range in kainic acid induced seizure model[55]. The maltol analogs showed a significant reduction in motor activity and prolonged hexobarbital induced sleeping time[56]. Humulene/Caryophyllene demonstrated anti-inflammatory activity via the modulation of the endocannabinoid system by attenuating the microglia induced release of pro inflammatory cytokines and chemokines[57]. The anxiolytic and antidepressant like effect of β-caryophyllene is reported in different animal models of anxiety and depression[58]. Furthermore, β-caryophyllene also exhibited neuroprotective activity against seizure-induced oxidative stress[59]. The compounds n-hexadecanoic acid demonstrated anti-inflammatory activity by competitively inhibiting phospholipase A2 (PLA2)[60]. Furthermore, hexadecanoic acid methyl ester (palmitic acid methyl ester) is demonstrated to be a novel neuroprotective compound in cerebral ischemia[61]. Squalene is reported to exhibit antioxidant and anti-inflammatory activities by an in vitro study conducted on murine peritoneal macrophages. Squalene targeted proinflammatory (inducible Nitric oxide synthas (iNOS), Cyclooxygenase-2 (COX-2), Nuclear factor kappa beta (NF-ĸB), Mitogen-activated protein kinases (MAPKs), Toll-like receptor 4 (TLR4), Matrix metalloproteinases (MMPs)) and anti-inflammatory (Nuclear factor E2-related factor 2 (Nrf2), Heme oxygenase 1 (HO-1), Proliferator-activated receptorgamma (PPARγ)) mediators and pathways in closely related phagocytic cells that cooperate during onset, progression and resolution of inflammation[62]. The compound α- linolenic acid (ALA) also known as 9,12,15-octadecatrienoic acid, exhibited psychotropic effects by modulating neurotransmission, anti-inflammation, anti-oxidation and neuroplasticity[63]. In a study, the anxiety like behavior in n-3 polyunsaturated fatty acid-deficient mice attributed to an alteration in cannabinoid receptor signaling in the prefrontal cortex and hypothalamus[64]. Linoleic acid (LA) and ALA in a ratio of 4:1 ( known as SR-3, 40 mg/kg ip) reduced PTZ and Carbamazepine induced seizure in a rat model[65,66].
MEAV could be used as an active chemical moiety; however, the daily recommended dose required to cross the blood brain barrier and protection against neurodegeneration needs to be determined by further in vitro studies. Further experiments are needed to evaluate the synergistic/antagonistic effect of the extract in the presence of xenobiotics. The in vivo studies needs to be coupled with further animal models to validate the findings. Though we have added both sexes, the sex differences could not be accounted for due to the low sample size and failure to monitor the estrous cycle.
MEAV displayed potential antiepileptic activity with increasing doses of Aloe vera, anxiolytic activity at 200 and 400 mg/kg and sedative activity at 800 mg/kg. The bioactive compounds responsible for such activities are humulene, squalene, β-caryophyllene, hexadecanoic acid, hexadecanoic acid methyl ester, alpha-tocopherol, maltol and phytol. The current therapies for long standing neurological disorders provide symptomatic relief but fail to address the core issue of neuronal damage. The MEAV derived compounds reported both in vitro and in vivo studies inhibit activation of oxidative and neuroinflammatory pathways, providing neuroprotection and improving behavioral phenotypes in neurological disorders. Hence, the MEAV derived compound could serve as an alternative treatment for neurological disorders by developing a better therapeutic formulation.
Conflict of interest:
The authors declared no conflicts of interest.
References
- Schiavone S, Jaquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal 2013;18(12):1475-90.
- Wise MG, Rundell JR. Anxiety and neurological disorders. Seminar clin Neuropsychiatry 1999;4(2):98-102.
- Kanner AM. Is major depression a neurologic disorder with psychiatric symptoms? Epilepsy Behav 2004;5(5):636-44.
- Yuen AWC, Keezer MR, Sander JW. Epilepsy is a neurological and a systemic disorder. Epilepsy Behav 2018;78:57-61.
- Schwartz M, Deczkowska A. Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol 2016;37(10):668-79.
- Samarh SN, Khalaf NA, Hajhamad MM. Evidence based medical use of Aloe vera extracts, short review of literature. Int J Res Med Sci 2017;5(10):4198-202.
- Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol 2008;53(4):163-6.
- Wang Y, Cao L, Du G. Protective effects of Aloe vera extract on mitochondria of neuronal cells and rat brain. Zhongguo Zhong Yao Za Zhi 2010;35(3):364-8.
- Yuksel Y, Guven M, Kaymaz B, Sehitoglu MH, Aras AB, Akman T, et al. Effects of Aloe Vera on Spinal Cord Ischemia-Reperfusion Injury of Rats. J Invest Surg 2016;29(6):389-98.
- Tabatabaei SR, Ghaderi S, Bahrami-Tapehebur M, Farbood Y, Rashno M. Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomed Pharmacother 2017;96:279-90.
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res 2005;39(10):1119-25.
- Salman M, Tabassum H, Parvez S. Tannic Acid Provides Neuroprotective Effects Against Traumatic Brain Injury Through the PGC-1alpha/Nrf2/HO-1 Pathway. Mol Neurobiol 2020;57(6):2870-85.
- Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 2008;3(3-4):115-26.
- Rathor N, Arora T, Manocha S, Patil AN, Mediratta PK, Sharma KK. Anticonvulsant activity of Aloe vera leaf extract in acute and chronic models of epilepsy in mice. J Pharm Pharmacol 2014;66(3):477-85.
- Abdollahnejad F, Mosaddegh M, Nasoohi S, Mirnajafi-Zadeh J, Kamalinejad M, Faizi M. Study of Sedative-Hypnotic Effects of Aloe vera L. Aqueous Extract through Behavioral Evaluations and EEG Recording in Rats. Iran J Pharm Sci 2016;15(1):293-300.
- Zhu HL, Wan JB, Wang YT, Li BC, Xiang C, He J, et al. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia 2014;55(1):3-16.
- Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content and antioxidant activity of Limnophila aromatica. J Food Drug Anal 2014;22(3):296-302.
- Sruthi DR, Indira G. A comparative evaluation of maceration, soxhlation and ultra sound assisted extraction for the phytochemical screening of the leaves of Nephelium lappaceum L. (Sapindaceae). J Pharmacogn Phytochem 2016;5(5):386-9.
- Britton G. Methods in Plant Biochemistry. London: Academic Press 1991:473-78p.
- Saritha, Anilakumar K. Toxicological evaluation of methanol extract of Aloe vera in rats. Int J Pharma Bio Sci 2010;1(5):142-9.
- Ashafa AO, Sunmonu TO, Abass AA, Ogbe AA. Laxative potential of the ethanolic leaf extract of Aloe vera (L.) Burm. f. in Wistar rats with loperamide-induced constipation. J Nat Pharm 2011;2(3):158-62.
- Burstein AH, Cox DS, Mistry B, Eddington ND. Phenytoin pharmacokinetics following oral administration of phenytoin suspension and fosphenytoin solution to rats. Epilepsy Res 1999;34(2-3):129-33.
- Shastry R, Ullal SD, Karkala S, Rai S, Gadgade A. Anxiolytic activity of aqueous extract of Camellia sinensis in rats. Indian J Pharmacol 2016;48(6):681-6.
- Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C 2006;24(1):103-54.
- Soumaya KJ, Dhekra M, Fadwa C, Zied G, Ilef L, Kamel G, et al. Pharmacological, antioxidant, genotoxic studies and modulation of rat splenocyte functions by Cyperus rotundus extracts. BMC Complement Altern Med 2013;13(1):1.
- Forouzanfar F, Ghazavi H, Vahedi MM, Tarrah K, Yavari Z, Hosseini A, et al. Tanacetum parthenium enhances pentobarbital-induced sleeping behaviors. Avicenna J Phytomed 2020;10(1):70-7.
- Shah SM, Sadiq A, Shah SM, Ullah F. Antioxidant, total phenolic contents and antinociceptive potential of Teucrium stocksianum methanolic extract in different animal models. BMC Complement Altern Med 2014;14(1):1-7.
- Giardina WJ, Gasior M. Acute seizure tests in epilepsy research: electroshock- and chemical-induced convulsions in the mouse. Curr Protoc Pharmacol 2009;45(1):5-22.
- Florek-Luszczki M, Wlaz A, Kondrat-Wrobel MW, Tutka P, Luszczki JJ. Effects of WIN 55,212-2 (a non-selective cannabinoid CB 1 and CB 2 receptor agonist) on the protective action of various classical antiepileptic drugs in the mouse 6 Hz psychomotor seizure model. J Neural Transm 2014;121(7):707-15.
- Vogel HG, editor. Drug discovery and evaluation: pharmacological assays. SSBM 2002:487.
- Svensson TH, Thieme G. An Investigation of a New Instrument to Measure Motor Activity of Small Animals. Psychopharmacologia 1969;14(2):157-63.
- Dey P, Chandra S, Chatterjee P, Bhattacharya S. Neuropharmacological properties of Mikania scandens (L.) Willd. (Asteraceae). J Adv Pharm Technol Res 2011;2(4):255-9.
- Thiebot MH, Soubrie P, Simon P, Boissier JR. Dissociation of two components of rat behaviour by psychotropic drugs. Utilization for studying anxiolytic drugs. Psychopharmacologia 1973;31(1):77-90.
- Cherksey BD, Altszuler N. On the mechanism of potentiation by morphine of thiopental sleeping time. Pharmacol 1974;12(6):362-71.
- Ghosh G, Panda P, Rath M, Pal A, Sharma T, Das D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn Res 2015;7(1):110-3.
- Hadizadeh F, Rahimi B, Taghiabadi E, Razavi M, Karimi G. Evaluation of anticonvulsant effect of two novels 4-[1-(4-fluorobenzyl)- 5-imidazolyl] dihydropyridine derivatives in mice. Res Pharm Sci 2013;8(2):91-5.
- Simiand J, Keane PE, Morre M. The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacol 1984;84(1):48-53.
- Wichniak A, Wierzbicka A, Walecka M, Jernajczyk W. Effects of Antidepressants on Sleep. Curr Psychiatry Rep 2017;19(9):1-7.
- Sultana N, Najam R. Anxiolytic activity of Aloe vera (L.) Burm. F tested in rodents. Pak J Pharmacol 2012;29(1):7-15.
- Kumar R, Jehan M, Sahu M. Evaluation of antianxiety activity of lyophilized Aloe vera succulent in Albino Swiss mice. Indian J Appl Res 2014;4.
- Donthula kk, Dholi S. Evaluation of antipsychotic and anxiolytic activity of Aloe vera (Aloe barbadensis miller) in rats. World J Pharm Res 2018;7:889.
- Aguiar CC, Almeida AB, Araujo PV, de Abreu RN, Chaves EM, do Vale OC, et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev 2012;2012:795259.
- Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol 2014;5:532.
- Nieoczym D, Albera E, Kankofer M, Wlaz P. Maximal electroshock induces changes in some markers of oxidative stress in mice. J Neural Transm 2008;115(1):19-25.
- Rong YQ, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A 1999;96(17):9897-902.
- Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol 2007;564(1-3):146-9.
- Krol ES, Kramer-Stickland KA, Liebler DC. Photoprotective actions of topically applied vitamin E. Drug Metab Rev 2000;32(3-4):413-20.
- Popa O, Babeanu NE, Popa I, Nita S, Dinu-Parvu CE. Methods for obtaining and determination of squalene from natural sources. Biomed Res Int 2015;2015:367202.
- Costa JP, Ferreira PB, De Sousa DP, Jordan J, Freitas RM. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci Lett 2012;523(2):115-8.
- Costa JP, de Oliveira GA, de Almeida AA, Islam MT, de Sousa DP, de Freitas RM. Anxiolytic-like effects of phytol: possible involvement of GABAergic transmission. Brain Res 2014;1547:34-42.
- Silva RO, Sousa FB, Damasceno SR, Carvalho NS, Silva VG, Oliveira FR, et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam Clin Pharmacol 2014;28(4):455-64.
- Ulatowski L, Parker R, Warrier G, Sultana R, Butterfield DA, Manor D. Vitamin E Is Essential for Purkinje Neuron Integrity. Neuroscience 2014;260:120-9.
- Ambrogini P, Minelli A, Galati C, Betti M, Lattanzi D, Ciffolilli S, et al. Post-seizure alpha-tocopherol treatment decreases neuroinflammation and neuronal degeneration induced by status epilepticus in rat hippocampus. Mol Neurobiol 2014;50(1):246-56.
- Hong S, Iizuka Y, Lee T, Kim CY, Seong GJ. Neuroprotective and neurite outgrowth effects of maltol on retinal ganglion cells under oxidative stress. Mol Vis 2014;20:1456-62.
- Kim YB, Oh SH, Sok DE, Kim MR. Neuroprotective effect of maltol against oxidative stress in brain of mice challenged with kainic acid. Nutr Neurosci 2004;7(1):33-9.
- Kimura R, Matsui S, Ito S, Aimoto T, Murata T. Central depressant effects of maltol analogs in mice. Chem Pharm Bull 1980;28(9):2570-9.
- Klauke AL, Racz I, Pradier B, Markert A, Zimmer AM, Gertsch J, et al. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharm 2014;24(4):608-20.
- Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S. Beta-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav 2014;135:119-24.
- Liu H, Song Z, Liao D, Zhang T, Liu F, Zhuang K, et al. Neuroprotective effects of trans-caryophyllene against kainic acid induced seizure activity and oxidative stress in mice. Neurochem Res 2015;40(1):118-23.
- Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des 2012;80(3):434-9.
- Lee RH, e Silva AC, Possoit HE, Lerner FM, Chen PY, Azizbayeva R, et al. Palmitic acid methyl ester is a novel neuroprotective agent against cardiac arrest. Prostaglandins Leukot Essent Fatty Acids 2019;147:6-14.
- Cardeno A, Aparicio-Soto M, Montserrat-de la Paz S, Bermudez B, Muriana FJG, Alarcon-de-la-Lastra C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J Funct Foods 2015;14:779-90.
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010;68(2):140-7.
- Larrieu T, Madore C, Joffre C, Laye S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J Physiol Biochem 2012;68(4):671-81.
- Yehuda S, Carasso RL, Mostofsky DI. Essential fatty acid preparation (SR-3) raises the seizure threshold in rats. Eur J Pharmacol 1994;254(1-2):193-8.
- Taha AY, Filo E, Ma DW, McIntyre Burnham W. Dose-dependent anticonvulsant effects of linoleic and alpha-linolenic polyunsaturated fatty acids on pentylenetetrazol induced seizures in rats. Epilepsia 2009;50(1):72-82.