- Corresponding Author:
- A. Jain
Lecturer, B. R. Nahata College of Pharmacy, Mandsaur - 458 001, India
E-mail: avijeet_9826275340@rediffmail.com
| Date of Submission | 15 July 2005 |
| Date of Revision | 14 March 2006 |
| Date of Acceptance | 26 November 2006 |
| Indian J. Pharm. Sci., 2006, 68 (6): 740-743 |
Abstract
Ethanol extract of leaves and flavonoid (isolated from leaves extract) from Tephrosia purpurea were evaluated for hepatoprotective activity in rats by inducing hepatotoxicity with carbon tetrachloride. These fractions were administered orally at a dose of 100 mg/kg/day. Serum level of transaminases, alkaline phosphate, and total bilirubin were used as biochemical markers of hepatotoxicity. Histopathological changes in the liver were also studied. The results of the study indicated that the hepatoprotective activity was more in ethanolic extract of leaves than isolated flavonoid.
Flavonoids, which occur both in the free state and as glycosides, are the largest group of naturally occurring phenols. More than 2000 of these compounds are now known, with nearly 500 occurring in free state. They are formed from three acetate units and phenylpropanoid units. They are widely distributed in nature but are more common in young tissues, where they occur in cell sap. They have been used extensively as chemotaxonomic markers and are abundant in the polygonaceae, rutaceae leguminosae, umbelliferae, and compositae. Although, the original high hopes for the therapeutic usefulness were not immediately realized but recently researchers have demonstrated their involvement in the medicinal action of drugs, such as, liquorice root and gingko. It is very probable that a number of herbal remedies, whose constituent are yet unknown, will be shown to contain active flavonoids [1]. Flavonoids possessing broad biological properties have been demonstrated. The capability to interact with protein phosphorylation and the antioxidant, iron-chelating, and free radical scavenging activity may account for the wide pharmacological profile of flavonoids [2]-[4] .
Tephrosia purpurea Pers. is a pantropical coastal shrub that grows up to 1 m in height [5]. It occurs throughout the Indian sub continent. Previous phytochemical investigation on this plant have shown the presence of coumarins [6] flavonoids and rotenoids [7],[8], flavanones [9],[10] and isoflavanones [11] and quercetin [12].
Materials and Methods
The plant of Tephrosia purpurea was collected from the forest around Dr. H. S. Gour Vishwavidyalaya, Sagar in the month of September and identified at Department of Botany, Dr. H. S. Gour Vishwavidyalaya, Sagar (Voucher no T-254).
Extraction of drug
Plant leaves were selected for assessment of the activity. The dried leaves (dried under the shade) were extracted with 95% ethanol for 48 hour after defatting with petroleum ether for 72 hour. The extracts were filtered and concentrated in vacuum under reduced pressure and dried in desicator at room temperature.
Isolation of flavonoid
Isolation of flavonoid from the ethanol extract of leaves was carried out on the basis of solubility. For isolation, distilled water (100 ml) was added to the concentrated ethanol extract (50 ml). After about 1 hour precipitation was observed. This precipitate was recovered by filtration. Further, the precipitate was dissolved in chloroform (100 ml) by shaking for 15 min and heated gently for 5 min and filtered in hot state. The chloroform soluble fraction was discarded and insoluble fraction, left on filter paper was dissolved in ethyl acetate (100 ml) by shaking for 15 min and heated gently for 5 min and filtered in hot state. The ethyl acetate soluble fraction was discarded and insoluble fraction, left on filter paper was crystallized with methanol, thereafter the residue obtained was designated as fraction A.
Characterization of fraction A
For characterization qualitative tests and spectral studies were carried out. Shinoda test (qualitative test) was positive which is characteristic of flavonoids. Qualitative tests for other chemical constituents were found negative. The UV spectroscopy of the compound was performed using Shimadzu. The λmax of the compound was found at 208.9, 256.6 and 355.2 nm. The U.V. maxima indicate the possibility of flavonoidal residue supported by the literature [13]. The IR spectrum of the fraction A showed strong absorption bands at 3415 cm-1 (–OH), 1602.1 cm-1 (unsaturated C = O), 2933 cm-1 (C-Me), 1060 cm-1 (glycosidic C – O) groups that are found in the flavonoids [14],[15]. So these different studies showed that isolated fraction A is containing flavonoid.
Assessment of hepatoprotective activity
A prior permission was taken from Animal Ethical Committee to carry out the activity (Approval no.216/01/AB/CPCSEA and date-15 Jan 2004). Solution of CCl4 (50%) was prepared in liquid parraffin for use as challenge. Ethanol extract of leaves and fraction A were suspended in 2% PVP solution (10 mg/ml of drug in each case) for administration. The experimental animals (Wister rats of either sex, weighing between 100-150 g) were divided into 4 groups of each. The group I served as control. The group 2-4 recieved 2ml/kg/day oral dose of ethanol extract of leaves and fraction A, respectively. This treatment was carried out for 4 days. On the 5th day, blood samples were collected through retro orbital plexus with the help of thin capillaries into Epphendorf tubes and allowed to coagulate for 30 min. Clear serum was separated and the estimation of SGOT (serum glutamate oxaloacetate transaminase), SGPT (serum glutamate pyruvate transaminase)[16], SALP (serum alkaline phosphate)[17] and total bilirubin [18] were undertaken using RA- 50 model analyzer (Bayer India Ltd.).
Histological investigation
The livers were excised from the experimental animals of each group after collecting the blood sample and washed with normal saline. Initially the materials were fixed in 10% neutral formalin solution. Sections were cut using microtome technique [19] after paraffin embedding. The sections were processed in alcohol xylene series and were stained with hematoxylin and eosin. The different sections were examined microscopically for the evaluation of histopathological changes.
Statistical analysis
C – O) groups that are found in the flavonoids14,15. So Results of liver functioning biochemical parameters were reported as mean ± SD of six animals in each group. student's t-test was used for determining significance.
Results
The results are presented in Table 1 Rats treated with CCl4 alone developed significant hepatocellular damage as evidenced from increase in serum level of SGOT, SGPT, SALP and bilirubin when compared with control (Table 1). The samples of the Group II animals showed drastic increase in the levels of SGOT (940.33 ± 15.25), SGPT (575.24 ± 12.36), SALP (775.20 ± 13.57) and total bilirubin (3.59 ± 1.33). Administration of ethanolic extracts of leaves and fraction. A caused reduction in increased serum level of SGOT, SGPT, SALP, and total bilirubin significantly.
Histopathology
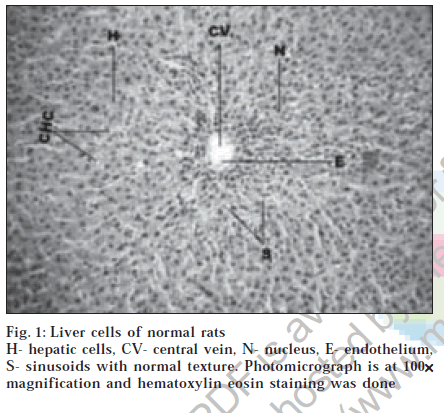
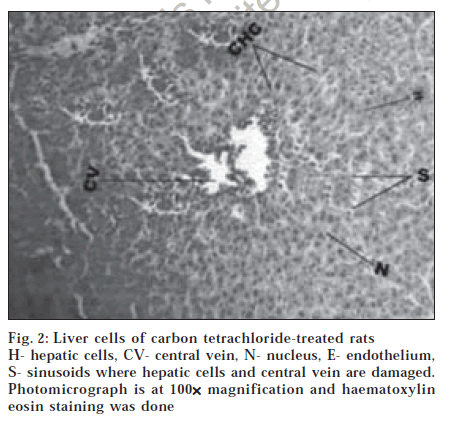
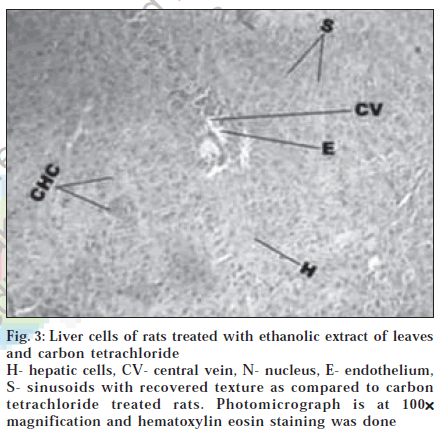
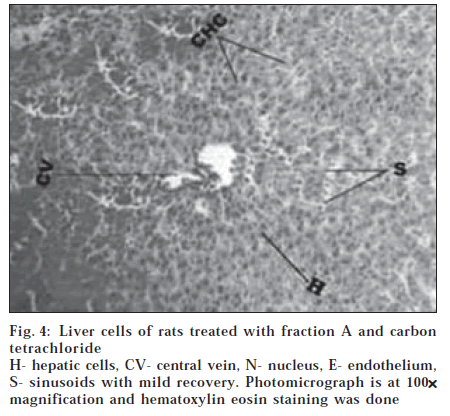
Histological section of control animals Group I showed normal hepatic cells with well-preserved cytoplasm, prominent nucleus and nucleolus and conspicuous central vein (fig. 1). Histological section of Group II animals showed high degree of damage characterized by cell vacuolation, pyknotic and degenerated nuclei and damage to wall of capillaries (fig. 2). Histopathological profile of the Group III showed the recovery against the CC14 induced damage as compared to control (fig. 3). Whereas, the histological section of the Group IV showed that nuclei are not clear as in normal hepatocytes but as compared to the CCl4 damage ones, the hepatocytes with normal nucleus are more. Endothelium was disrupted at places but in lesser number than CCl4 intoxicated rats. Hepatic cells adjoining to interlobular vein showed atrophy. Pyknotic nucleus and vacuolation in cytoplasm were observed to be low. There seemed to be a satisfactory recovery (fig. 4).
| Groups | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| SGOT (AST) (µ/L) | SGPT (ALT) (µ/L) | SALP (µ/L) | Total Bilirubin (mg/L) | ||||
| Normal | 240.13 ± 9.24 | 170.25 ± 4.23 | 558.36 ± 11.35 | 1.26 ± 0.65 | |||
| CC1 4 treated | 940.33 ± 15.25 | 575.24 ± 12.36 | 775.20 ± 13.57 | 3.59 ± 1.33 | |||
| Leaves Extract | 390.42 ± 6.24* | (78.57) | 235.31 ± 5.05* | (83.95) | 588.39 ± 10.23* (86.15) | 1.47 ± 0.92* | (90.98) |
| Fraction A | 645.12 ± 7.28* | (42.14) | 430.12 ± 6.05* | (34.56) | 421.22 ± 7.32* (70.96) | 1.89 ± 0.98* | (72.96) |
Table 1: Serum Parameters Of Animals Treated With Cci4, Ethanolic Extract Of Leaves And Fraction A.
Figure 3:Liver cells of rats treated with ethanolic extract of leaves and carbon tetrachloride H- hepatic cells, CV- central vein, N- nucleus, E- endothelium, S- sinusoids with recovered texture as compared to carbon tetrachloride treated rats. Photomicrograph is at 100×××××magnification and hematoxylin eosin staining was done.
Discussion
Results indicated that the ethanolic extracts of leaves and fraction A from T. purpurea provided significant protection against the toxic effect of CCl4 on liver. Preventive action of liver damage induced by the CCl4 has widely been used as indicator of the liver protective in general [20]. CC14 produces an experimental damage that histologically resembles viral hepatitis [21]. Toxicity begins with the change in endoplasmic reticulum, which results in the loss of metabolic enzymes located in the intracellular structures [22].
The toxic metabolite CC13 radical is produced by microsomal oxidase system, binds covalently to the macromolecule and causes peroxidative degradation of lipid membrane of the adipose tissue. In view of this, the test dug mediated reduction in levels of SGOT and SGPT towards the respective normal values is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage caused by CC14. This effect is agreement with the commonly accepted view that serum level of transaminases return to normal with the healing of hepatic parenchyma and regeneration of hepatocytes [23]. Alkaline phosphate is the prototype of these enzymes that reflects the pathological alteration in biliary flow [24]. CCl4 induced elevation of this enzymatic activity in the serum is in line with high level of serum bilirubin content. The test drug mediated suppression of the increased SALP activity with the concurrent depletion of raised bilirubin suggest the possibility of the test drug being able to stabilize biliary dysfunction in rat liver during hepatic injury with CCl4.
Histopathological studies reveal the changes, which take place during the damage and recovery. The test drug mediated recovery supports the hepatoprotective activity of the same. Llavarasan et al.25 have reported such type of histopathological evidences on restoring the damage of liver cells.
The results of this investigation indicated that the ethanol extract of leaves and fraction A(isolated from leaves extract) from Tephrosia purpurea significant hepatoprotective activity against CCl4 induced liver damage in rats. As fraction A is a Flavonoid and it has shown hepatoprotective activity it is concluded that hepatoprotective activity in the drug is due to presence of flavonoids.the higher activity of leaves extract may be contributed due to synergistic effect of floavonoids present in the drug.
References
I thank the AICTE, New Delhi from heart for providing scholarship during my M. Pharm dissertation work.
References
- Trease, G.E. and Evans, W.C., In; Pharmacognosy, 14th Edn., W.B. Saunders Company, London, 249.
- Saija, A., Scalese, M., Lanza, M., Marzullo, D. and Bonina, F., Free Rad. Biol. Med., 1995, 19, 481.
- Saija, A., Tomaino, A., Trombetta, D., Giacchi, M., Pasquale, A.D. and Bonina, F., Int. J. Pharm., 1998,175, 85.
- Van Acker, S.A., Van Den Berg D.J. and Tromp, M.N., Free Rad. Biol. Med., 1996, 20, 331.
- Perry, L. M., In; Medicinal plants of East and South East Asia, MIT Press, Cambridge, 1980, 620.
- Rajani, P. and Sarma, P.N., Phytochemistry, 1988, 27, 648.
- Vankata, R.E. and Ranga, R., Phytochemistry, 1984, 23, 2339.
- Vankata, R.E. and Ranga, R., Phytochemistry, 1979, 18, 1581.
- Husaini, F.A. and Shoeb, A., Planta Med., 1986, 52, 220.
- Gupta,R.A., Krishnamurti,M. and Parthasarathi,J. Phytochemistry, 1980, 19, 1264.
- Rao, E.V., Murthy, M.S.R. and Ward, R.S., Phytochemistry, 1984, 23, 1493.
- Khanna, P., Kamal, R. and Jain, S.C., Sci. Cult., 1977, 43, 396.
- Harborne, J.B., In; Phytochemical Methods, 1st Edn., Chapman and Hall London, 1973, 70.
- Kalsi, P.S., In; Spectroscopy of Organic Compounds, 5th Edn., New Age Publisher, Mumbai, 2002, 88.
- Masbry, T.J. and Markhan, K.P., In; The Systematic Identification of Flavonoids, Springer Verlag, Berlin, 1970, 41.
- Reitman, S. and Frankel, A. S., Amer. J. Clin. Pathol., 1957, 28, 56.
- Chemie D.G.F.K., J. Clin. Chem., 1970, 8, 658.
- Jendrassik, L. and Grof, P., Biochem. Z., 1938, 81, 297.
- Galigher, A.E. and Kayloff, E.N., Philadelphia, 1971, 77.
- Clausion, G.A., Pathol. Immunopathol. Res., 1989, 8, 104.
- James, G.W.L. and Pickering, R.W., Drug Research, 1976, 26, 2197.
- Recnagel, R. O., Trends Pharmacol. Sci., 1983, 4, 129.
- Thabrew, M.I., Joice, P.D.T.M. andRajatissa, W.A., PlantaMedica, 1987, 53, 239.
- Ploa, G.L. and Hewitt, W.R., In; Principle and Methods of Toxicology, 2 ndEdn., Raven Press, NewYork,1989,399.
- Llavarasan, R., Mohideen, S., Vijayalakshmi, M. and Manamnam G., Indian J. Pharm. Sci., 2001, 63, 504.