- *Corresponding Author:
- Abdelbagi Alfadil
Department of Clinical Microbiology and Immunology, King Abdulaziz University, Jeddah 21589, Saudi Arabia
E-mail: Indian J Pharm Sci 2024;86(4):1492-1500
| Date of Received | 02 April 2024 |
| Date of Revision | 29 July 2024 |
| Date of Acceptance | 12 August 2024 |
| Indian J Pharm Sci 2024;86(4):1409-1417 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the pharmacokinetics of 2,3-dimethylquinoxaline using web server (absorption, distribution, metabolism, and excretion), safety profile. Moreover, to assess the efficacy of 2,3-dimethylquinoxaline (a natural quinoxaline derivative) in vitro and in vivo. The absorption, distribution, metabolism, and excretion of 2,3-dimethylquinoxaline were predicted using the web servers Swiss absorption, distribution, metabolism, and excretion and ProTox-II, respectively. In vitro efficacy against Madurella mycetomatis and a wide range of other pathogenic fungi was determined by microdilution assay. In vivo activity was evaluated using a topical gel (1 %) in the Bagg Albino mice eumycetoma model and an oral candidiasis model. 2,3-dimethylquinoxaline showed favourable absorption, distribution, metabolism, and excretion drug-likeness features and a high safety profile. It inhibited Madurella mycetomatis at a minimum inhibitory concentration of 312 µg/ml and exhibited potent in vitro activity against a range of other fungi, including Cryptococcus neoformans (minimum inhibitory concentration=9 µg/ml) and Candida tropicalis is (minimum inhibitory concentration=1.125 µg/ml). In vivo, 2,3-dimethylquinoxaline gel was effective in treating both skin Madurella mycetomatis infection and oral candidiasis. 2,3-dimethylquinoxaline is a promising natural antifungal drug with the potential to treat eumycetoma and other fungal infections.
Keywords
2,3-dimethylquinoxaline, quinoxaline, antifungal, Madurella mycetomatis, Candida tropicalis
Fungal infections are a major global health problem, affecting millions of people each year and causing significant morbidity and mortality. They can range in severity from mild and superficial infections of the skin, hair, and nails to life-threatening invasive infections of the lungs, brain, and other organs. Invasive fungal infections can be fatal, especially in people with weakened immune systems[1].
The most common and life-threatening fungal infections are caused by Candida, Aspergillus, and Cryptococcus[2,3]. Mycetoma is a particularly challenging fungal infection that is endemic in the tropics and subtropics[4]. Limitations of current mycetoma treatment include; long duration of treatment, high risk of recurrence, side effects of medication limited efficacy expense, and lack of accessibility[5].
Most of the current antifungal drugs have one or more of the following significant limitations; severe adverse effects, parenteral administration e.g. polyenes, drug interactions[6]. Emerging resistance, relatively high cost of some antifungal formulations e.g. liposomal amphotericin[7] and limited skin permeability[8,9].
Quinoxaline derivatives are a versatile class of natural and synthetic compounds with a wide range of biological activities, including antimicrobial activity. Their ease of synthesis in the laboratory makes them a promising platform for the discovery of new antimicrobial drugs[10]. 2,3-Dimethylquinoxaline (DMQ) is a natural compound found in the Chromolaena odorata plant[11]. It is available commercially at a reasonable price. It has been shown to have antimicrobial activity, but its antifungal potential and pharmacological profile have not been fully explored[12].
This study aims to comprehensively study the pharmacokinetic, safety, in vitro, and in vivo antifungal potential of DMQ against different fungal infection. The results of this study could pave the way for the development of DMQ into a novel antifungal drug with a favourable pharmacological profile.
Materials and Methods
DMQ, identified by CAS number 2379-55-7, is provided by Sigma-Aldrich in Taufkirchen, Germany (Catalog No: D184977). Hydroxypropyl Methylcellulose (HPMC) and all other components are of pharmaceutical-grade quality.
Bacterial strain, growth condition and medium:
A clinical strain of the fungus Madurella mycetomatis (MM) was included in this study. This fungal isolate was cultured and kept on Sabouraud’s Dextrose Agar (SDA) at a temperature of 37°. A total of 73 clinical isolates, featuring a broad range of pathogenic fungi, including yeasts such as Candida albicans (C. albicans), C. parapsilosis, C. glabrata, C. krusei, C. auris, C. tropicalis, C parapsilosis, Cryptococcus neoformans, and molds including Aspergillus fumigatus, Aspergillus niger, and Trichophyton mentagrophytes, were employed in in vitro experiments. For both in vitro and in vivo investigations, a reference strain from the American Type Culture Collection (ATCC), C. albicans ATCC 10231, was utilized.
Plain and medicated DMQ hydrogel:
DMQ hydrogel refers to a hydrogel that contains a compound called 2 DMQ as one of its components. Hydrogels are three-dimensional, they can hold a significant amount of water while maintaining their structural integrity. They are commonly used in various medical and pharmaceutical applications due to their unique properties, including high water content and biocompatibility. In order to prepare plain hydrogel, 1 g of HPMC powder was added to an appropriate beaker and mixed with 49 g of purified water. The beaker was covered and left for 24 h at room temperature to allow the gel to form. To formulate DMQ hydrogel at 1 % w/w, 0.5 g of DMQ were dissolved in 4.5 g of methanol to make a final concentration of 100 mg/g. The drug solution in methanol was then gradually added to 45 g of the plain HPMC gel, with continuous gentle mixing. Both plain and medicated gels were stored in suitable umber glass jars at 8o[13].
In silico prediction of Absorption, Distribution, Metabolism and Excretion (ADME):
As they are a crucial aspect of drug discovery and development. It involves the use of computational methods and modelling techniques to predict how a potential drug compound will behave within the human body, with a particular focus on its pharmacokinetics and potential toxicological effects. Thus, the SDF file for DMQ (CID16925, SMILES notation: CC1=NC2=CC=CC=C2N=C1C) was acquired from PubChem. SwissADME was employed for predicting its pharmacokinetic and associated parameters[14]. Toxicological data prediction was carried out using ProTox II[15].
In vitro antifungal susceptibility testing:
To assess the efficacy of DMQ in vitro, broth microdilution assay was used. In accordance with the guidelines set forth by the Clinical and Laboratory Standards Institute (CLSI), we conducted in vitro testing to assess the susceptibility of the pathogen to antifungal agents. To initiate this process, we first prepared a stock solution of the antifungal drug in Dimethyl Sulfoxide (DMSO). This concentrated solution was then meticulously diluted within Roswell Park Memorial Institute (RPMI)-1640 medium to generate a range of concentrations spanning from 0.303 to 312 μg/ml.
Concurrently, a suspension of the clinical isolate (referred to as MM) was meticulously prepared in RPMI-1640 medium and adjusted to a specific concentration of 104 cells/ml. Subsequently, 100 μl of this fungal suspension was dispensed into each well of a 96-well microtiter plate, followed by the addition of 100 μl of the antifungal drug solution into each respective well. The microtiter plate was carefully placed into an incubator, set to maintain a constant temperature of 37°, and left undisturbed for a duration of 24 h-36 h. After this incubation period, a visual examination of the microtiter plate was conducted to assess any signs of fungal growth. The Minimum Inhibitory Concentration (MIC) was determined as the lowest concentration of the antifungal drug that effectively inhibited 50 % of fungal growth[16,17].
In vivo activity against MM:
The experimental protocol was approved by the Biomedical Ethics Committee at King Abdulaziz University (KAU) and the National Committee of Bioethics (NCBE) (Registration No: HA-02-J-008). All animal experiments were conducted in strict accordance with the ethical guidelines for animal care and use established by KAU and NCBE.
Animal model for infection by MM:
In this research, six male BALB/c mice, aged (6-8) w, were employed. Mice were purchased from faculty of pharmacy in KAU animal house and placed in specific-pathogen-free conditions with food and water.
A subcutaneous injection of 0.4 ml of 5×106 cfu MM suspension was administered into the neck of each mouse. The injection site was examined daily over a 2 w period to detect any lesion formation. Once MM infection was confirmed, a topical application of 1 % DMQ gel was provided to the lesion once a day, and the mice's lesions were subject to daily monitoring[18].
Animal model for oral candidiasis:
To assess the efficacy of the antifungal drug in vivo, a mouse model was used. The experimental procedure received official approval from both the Biomedical Ethics Committee at KAU and the NCBE, as evidenced by Registration No: HA-02-J-008. Every aspect of the animal experiments strictly adhered to the ethical principles and guidelines for animal care and use, as set forth by KAU and NCBE.
To assess the efficacy of DMQ gel against oral candidiasis, we employed a previously reported mouse model for the disease with minor adjustments[19]. Ten male BALB/c mice, aged 8 w, were selected for the development of oral candidiasis. Immunosuppression was induced in these mice with prednisolone (100 mg/kg subcutaneously) (Sigma Aldrich-P6004, Taufkirchen, Germany), 1 d before and 3 d after being infected with C. albicans ATCC 10231. Additionally, tetracycline hydrochloride solution at a concentration of 0.9 mg/ml (Sigma Aldrich-T7660, Taufkirchen, Germany) was administered orally to the mice starting 1 d before the infection.
Oral infection was initiated by swabbing the entire oral cavity of the mice with sterile cotton pads (baby cotton buds from Johnson and Johnson) saturated with a cell suspension (2×108 CFU/ml) of C. albicans ATCC 10231. The severity of the infection was assessed on a daily basis by evaluating the presence and extent of whitish, curd-like patches on the tongue surface.
After establishing the model, the mice were randomly divided into two groups, each consisting of 5 mice. One group received treatment with plain gel, while the other group was treated with a topical 1 % DMQ gel, administered daily for 7 d. The mice were closely monitored each day to determine whether they were cured or if their symptoms showed signs of improvement[18].
Results and Discussion
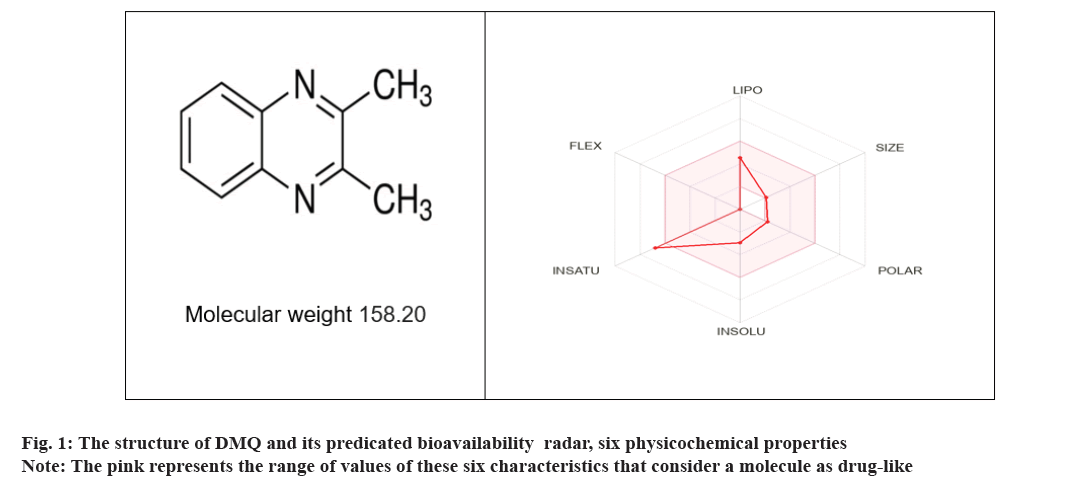
The structure of DMQ and its predicted bioavailability was presented in fig. 1, physiochemical properties drug-likeness ADME, and bioavailability score are presented in Table 1 and Table 2.
| MW (g/mol) | H-bond donors | H-bond acceptors | Rotatable bonds | Consensus Log P | TPSA ( Ų) | Water Solubility | Drug likeness role | ||
|---|---|---|---|---|---|---|---|---|---|
| Lipinski | Vebers | Egan | |||||||
| 158.20 g/mol | 0 | 2 | 0 | 2.09 | 25.78 Ų | Soluble or moderately soluble | Yes | Yes | Yes |
Note: MW=Molecular Weight; consensus log P=average of all predicted log Po/w and TPSA=Total Polar Surface Area
Table 1: Physiochemical properties, lipophilicity and water solubility drug-likeness of DMQ.
| GI absorption | BBB permeant | log Kp (cm/s) | CYP3A4 inhibitor | CYP2C19 inhibitor | CYP1A2 inhibitor | CYP2D6 inhibitor | CYP2C9 inhibitor | P-gp substrate | Bio availability score |
|---|---|---|---|---|---|---|---|---|---|
| High | Yes | -6.04 | No | No | Yes | No | No | No | 0.55 |
Note: GI=Gastrointestinal, BBB=Blood Brain Barrier and log Kp= skin permeation. For comparison Log Kp cm/s of betamethasone=-7.32, diclofenac acid=-4.98 and ketoconazole=-6.46
Table 2: ADME data and bioavailability.
In oral toxicity prediction; predicted Lethal Dose 50 (LD50) of 500 mg/kg, predicted toxicity class was 4, (accuracy 67 %). The toxicity model report of DMQ was shown in Table 3.
| Classification | Target | Prediction | Probability |
|---|---|---|---|
| Organ toxicity | Hepatotoxicity | Inactive | 0.71 |
| Toxicity endpoints | Carcinogenicity | Inactive | 0.68 |
| Immunotoxicity | Inactive | 0.99 | |
| Mutagenicity | Inactive | 0.5 | |
| Cytotoxicity | Inactive | 0.92 | |
| Tox21-nuclear receptor signaling pathways | Aryl hydrocarbon Receptor (AhR) | Inactive | 0.8 |
| Androgen Receptor (AR) | Inactive | 0.97 | |
| AR Ligand Binding Domain (AR-LBD) | Inactive | 0.88 | |
| Aromatase | Inactive | 0.8 | |
| Estrogen Receptor Alpha (ER) | Inactive | 0.81 | |
| Estrogen Receptor Ligand Binding Domain (ER-LBD) | Inactive | 0.98 | |
| Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) | Inactive | 0.79 | |
| Tox21-stress response pathways | Nuclear factor (erythroid-derived 2)-like 2/Antioxidant Responsive Element (Nrf2/ARE) | Inactive | 0.85 |
| Heat Shock factor response Element (HSE) | Inactive | 0.85 | |
| Mitochondrial Membrane Potential (MMP) | Inactive | 0.85 | |
| Phosphoprotein (tumor suppressor) p53 | Inactive | 0.72 | |
| ATPase family AAA Domain-containing protein 5 (ATAD5) | Inactive | 0.96 |
Table 3: Toxicity model report of DMQ.
The in vitro effectiveness of DMQ against MM was evaluated using the broth microdilution method. This approach involved testing various concentrations of DMQ to determine its MIC against MM. The findings revealed that the MIC of DMQ against MM was recorded at 312 µg/ml, equivalent to approximately 1.9 mM. This value signifies the concentration at which DMQ effectively inhibits the growth of MM in the test conditions.
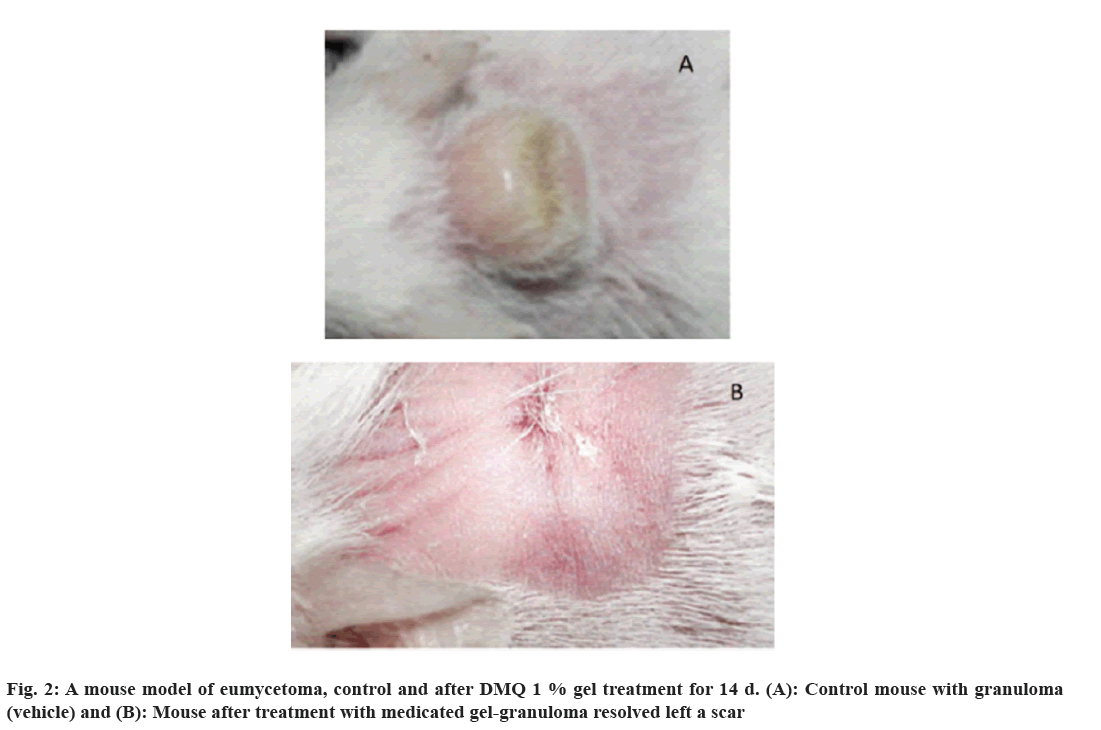
A mice model was employed to evaluate the efficacy of DMQ in combating eumycetoma, a chronic fungal infection. The study involved two groups; one served as the control, while the other received treatment with a 1 % DMQ gel over a 14 d period.
In the initial stages, the infected mice developed distinct granulomas on their necks, which persisted despite treatment with a plain gel, indicative of the challenging nature of eumycetoma. However, in stark contrast, the mice treated with DMQ 1 % gel exhibited a remarkable outcome. After the 14 d treatment regimen, the granulomas had completely disappeared, leaving behind only a minor scar. This observation suggests that DMQ holds promise as a therapeutic agent for effectively combating eumycetoma, a condition typically known for its persistent and treatment-resistant nature (fig. 2).
DMQ demonstrated a wide-ranging and comprehensive antifungal activity, MIC across a spectrum of fungal species. The MIC values varied between 9-1125 µg/ml when tested against these microorganisms (Table 4).
| Fungal species | MIC (µg/ml) | |
|---|---|---|
| 24 h | 48 h | |
| Crypt, neoformans | 9 | 9 |
| Aspergillus fumigatus | 370 | 370 |
| Aspergillus niger | 750 | 750 |
| C. albicans ATCC 10231 | 190 | 370 |
| C. albicans | 854 | 935 |
| C. auris | 280 | 370 |
| C. glabrata | 470 | 560 |
| C. krusei | 370 | 560 |
| C. parapsilosis | 560 | 750 |
| C. tropicalis | 935 | 1125 |
| Trichophyton mentagrophyte | 750 | 750 |
Table 4: In Vitro activity of DMQ against several fungal species.
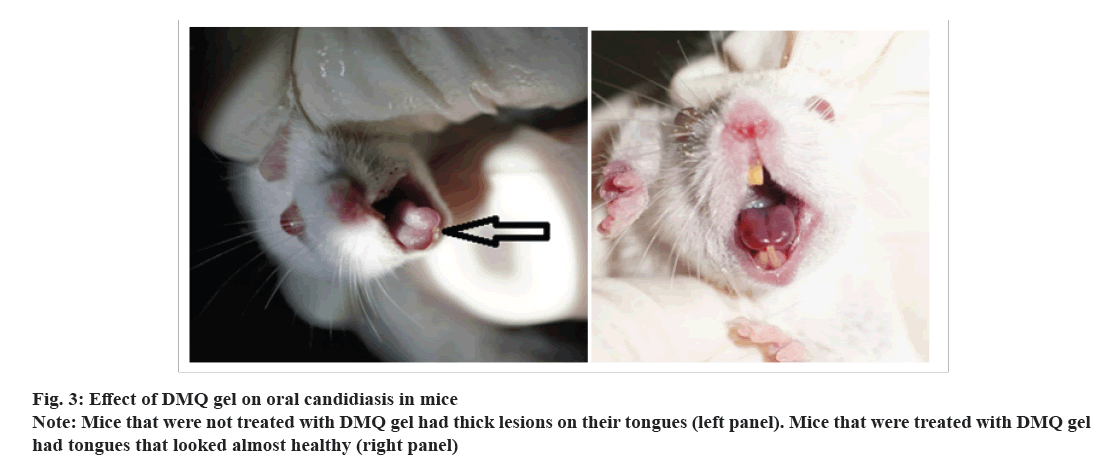
An examination of the mice model revealed a noteworthy trend in infection severity 3 d post-infection. Specifically, mice subjected to treatment with DMQ 1 % gel displayed a conspicuous reduction in infection severity when compared to the control group. Intriguingly, no viable Candida Colony-Forming Units (CFUs) were detected in the oral cavities of the mice treated with the medicated gel, indicating a potent antifungal effect.
Furthermore, an assessment of the dorsal tongue surfaces on 5 d post-infection presented an interesting observation. These surfaces exhibited a glossy and regular appearance in the group treated with DMQ 1 % gel (fig. 3), signifying a potential therapeutic impact of DMQ in maintaining the integrity of the dorsal tongue epithelium and countering the progression of Candida infection.
In silico ADME methods can help to screen compounds for drug-likeness of new compounds and optimize profile[20]. In the present study DMQ showed good Lipophilicity (LIPO) and skin permeation ability, suggesting its ability to reach target tissues at an appropriate concentration. In contrast, amphotericin B has minimal effect on MM, although it has excellent in vitro activity, explained by the limited drug distribution into the infected tissues[21].
In silico toxicology is essential to the evaluation of the toxicity and safety of substances as well as the process of developing new drugs. The capabilities and utility of computational techniques to predict toxicology continue to grow. These cutting-edge methodologies are used at different stages of the creation of a substance to forecast features that correlate with toxicity endpoints, construct and retrieve data from chemical databases, and model structure-activity relationships for new chemical formulations[22].
DMQ also showed a favourable toxicology profile, LD50 500 mg/kg, with potential hepatoxicity, carcinogenicity, immunotoxicity, mutagenicity among other enzymes pathways. Moreover, it doesn’t affect most Cytochrome P450 (CYP450) and P-glycoprotein (P-gp). Good water solubility, and bioavailability factor. These features made DMQ a good candidate for oral administering.
In silico predictions in this study suggest that DMQ inhibits the CYP1A2 enzyme. A few studies have shown that DMQ can induce the hepatic P-450 enzyme system[23,24].
It has been evaluated the mutagenicity potential of DMQ, along with 33 other quinoxaline and quinoline compounds, using the Salmonella/microsome assay[25]. Their results are consistent with our in silico predictions, which show that our tested compound has no risk of mutagenicity.
In addition to their safety profile, one of the most important characteristics of quinoxalines is their ability to reach target tissues at effective concentrations[26]. In contrast, amphotericin B has little or no value for treating deep-seated infections, despite its excellent in vitro activity. This is because amphotericin B does not distribute well into infected tissues[21].
The treatment of eumycetoma is challenging. It includes chronic therapy in addition to surgery. Itraconazole is usually indicated and terbinafine was considered as alternative drug with these antifungal plus surgery is low (25 %-30 %)[27]. In about 1/3rd of eumycetoma cases recurrence was documented[28]. Few studies suggested better clinical outcome with voriconazole or posaconazole, but low efficacy in case of liposomal amphotericin B[29]. Frequently patients develop extensive fibrosis, which retread the access of these antifungals to the infected tissues[30]. Regardless of variable efficacy of the aforementioned antifungals and taking into account the chronic nature of the disease, the patients likely suffer a long list of adverse effects associated with systemic use of azole antifungals or amphotericin B especially in patients with liver or renal impairment[19].
Given these issues, there is a rational need for antifungal medications with favourable pharmacokinetic and safety profiles antifungal agents in vitro high efficacy against MM, namely ravuconazole (MIC50 0.008 mg/l) and olorofim (MIC50 0.016 mg/l)[31,32]. Further studies with ravuconazole documented higher efficacy compared to itraconazole[32]. Olorofim inhibit pyrimidine biosynthesis pathway by targeting dihydroorotate dehydrogenase enzyme[33].
Extensive efforts of drug development for novel medication against MMleads to identifying 400 compounds effective at a concentration of 100 μM and 26 had an half-maximal Inhibitory Concentration (IC50) <8 µM. Of these compound 9 demonstrated in vivo efficacy[3]. Most studies focused on six compound series; aminothiazoles, phenothiazines, fenarimols, benzimidazoles ketoximes and antifolates[34]. Other promising antifungal against MM include; luliconazole and lanoconazole (median MICs 0.001-0.064 μg/ml for both) and ravuconazole (median MICs 0.008-2 μg/ml) fenbendazole and carbendazim (benzimidazole carbamates), tafenoquine (8-aminoquinolone derivative) and MMV1578570[35,36]. Few natural products were reported to showing in vitro activity against MM e.g. is a synthetic cinnamon oil blend (CIN-102), with MICs ranging from <32 μg/ml to 512 μg/ml[37].
The antibacterial activity of quinoxaline derivatives were reported[38]. In 2002, researchers discovered that quinoxalines have the potential to be effective antifungal drugs against Candida species[39]. Other studies have shown that synthetic quinoxalines with two substituents at positions 2 and 3 of the ring have excellent antifungal activity against fusarium oxysporum[40]. The inhibition of Topoisomerase II (Topo II) is one among other explanation for the mode of action of quinoxaline against eukaryotic organisms[41].
For the first time we showed that DMQ is a promising antifungal drug against Mycetoma and other fungal infections in vitro and in vivo, with excellent pharmacokinetic and safety profile. Further work is needed to identify its mechanism, in vivo pharmacokinetics. Further work is recommended to explore the molecular pathway of DMQ antifungal activity.
Conflict of interests:
The authors declared no conflict of interests.
References
- Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: Update 2012. Diagn Microbiol Infect Dis 2012;73(4):293-300.
[Crossref] [Google Scholar] [PubMed]
- José P, Alvarez-Lerma F, Maseda E, Olaechea P, Pemán J, Soriano C, et al. Invasive fungal infection in crtically ill patients: Hurdles and next challenges. J Chemother 2019;31(2):64-73.
[Crossref] [Google Scholar] [PubMed]
- Garber G. An overview of fungal infections. Drugs 2001;61(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Scolding P, Fahal A, Yotsu RR. Drug therapy for mycetoma. Cochrane Database Syst Rev 2018;2018(7).
- Welsh O, Salinas-Carmona MC, la Garza JA, Rodriguez-Escamilla IM, Sanchez-Meza E. Current treatment of mycetoma. Curr Treat Option Infect Dis 2018;10:389-96.
- Gubbins PO, Amsden JR. Drug-drug interactions of antifungal agents and implications for patient care. Expert Opin Pharmacother 2005;6(13):2231-43.
[Crossref] [Google Scholar] [PubMed]
- Micallef C, Aliyu SH, Santos R, Brown NM, Rosembert D, Enoch DA. Introduction of an antifungal stewardship programme targeting high-cost antifungals at a tertiary hospital in Cambridge, England. J Antimicrob Chemother 2015;70(6):1908-11.
[Crossref] [Google Scholar] [PubMed]
- Krysan DJ. The unmet clinical need of novel antifungal drugs. Virulence 2017;8(2):135-7.
- Erdal MS, Gurbuz A, Tan SB, Güngör S, Özsoy Y. In vitro skin permeation and antifungal activity of naftifine microemulsions. Turkish J Pharm Sci 2020;17(1):43.
[Crossref] [Google Scholar] [PubMed]
- Pereira JA, Pessoa AM, Cordeiro MN, Fernandes R, Prudêncio C, Noronha JP, et al. Quinoxaline, its derivatives and applications: A state of the Art review. Eur J Med Chem 2015;97:664-72.
[Crossref] [Google Scholar] [PubMed]
- González M, Cerecetto H. Quinoxaline derivatives: A patent review (2006-present). Expert Opin Ther Pat 2012;22(11):1289-302.
[Crossref] [Google Scholar] [PubMed]
- Elfadil A, Ali AS, Alrabia MW, Alsamhan H, Abdulmajed H, Abu II, et al. The wound healing potential of 2,3 dimethylquinoxaline hydrogel in rat excisional wound model. J Pharm Res Int 2023;35(8):1-8.
- Khedekar YB, Mojad AA, Malsane PN. Formulation, development, and evaluation of silymarin loaded topical gel for fungal infection. Int J Adv Pharm 2019;8(1):6-9.
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017;7(1):42717.
[Crossref] [Google Scholar] [PubMed]
- Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucl Acids Res 2018;46(1):257-63.
- Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics. Sci Transl Med 2012;4(126):126.
[Crossref] [Google Scholar] [PubMed]
- Mikkelsen K, Sirisarn W, Alharbi O, Alharbi M, Liu H, Nøhr-Meldgaard K, et al. The novel membrane-associated auxiliary factors AuxA and AuxB modulate β-lactam resistance in MRSA by stabilizing lipoteichoic acids. Int J Antimicrob Agents 2021;57(3):106283.
[Crossref] [Google Scholar] [PubMed]
- Halawi MH, Yassine W, Nasser R, Yusef H, Borjac J, Al Sagheer T, et al. Two weeks of chronic unpredictable stress are sufficient to produce oral candidiasis in balb/C mice. Asian J Microbiol Biotechnol Environ Sci 2020;22(2):254-64.
- Costa AC, Pereira CA, Junqueira JC, Jorge AO. Recent mouse and rat methods for the study of experimental oral candidiasis. Virulence 2013;4(5):391-9.
- Paul Gleeson M, Hersey A, Hannongbua S. In-silico ADME models: A general assessment of their utility in drug discovery applications. Curr Top Med Chem 2011;11(4):358-81.
[Crossref] [Google Scholar] [PubMed]
- Kloezen W, Parel F, Brüggemann R, Asouit K, Helvert-van Poppel M, Fahal A, et al. Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Med Mycol 2018;56(4):469-78.
[Crossref] [Google Scholar] [PubMed]
- Parthasarathi R, Dhawan A. In silico approaches for predictive toxicology. Vitr Toxicol 2018;91-109.
- Hahnemann B, Legrum W, Koss G, Netter KJ. Interactions of heterocyclic Maillard products with the hepatic microsomal monooxygenase system. Xenobiotica 1989;19(11):1319-26.
[Crossref] [Google Scholar] [PubMed]
- Beraud M, Gaillard D, Derache R. Effect of ingestion of derivatives of quinoxaline on the enzymatic detoxication activity of hepatic microsomes in the rat. Arch Int Pharmacodyn Ther 1975;218(2):328-37.
[Google Scholar] [PubMed]
- Hashimoto T, Negishi T, Namba T, Hayakawa S, Hayatsu H. Mutagenicity of quinoline derivatives and analogs-quinoxaline 1,4-dioxide is a potent mutagen. Chem Pharm Bull 1979;27(8):1954-6.
[Crossref] [Google Scholar] [PubMed]
- Ajani OO. Present status of quinoxaline motifs: Excellent pathfinders in therapeutic medicine. Eur J Med Chem 2014;85:688-715.
[Crossref] [Google Scholar] [PubMed]
- Sow D, Ndiaye M, Sarr L, Kanté MD, Ly F, Dioussé P, et al. Mycetoma epidemiology, diagnosis management, and outcome in three hospital centres in Senegal from 2008 to 2018. PloS One 2020;15(4):e0231871.
[Crossref] [Google Scholar] [PubMed]
- Wadal A, Elhassan TA, Zein HA, Abdel-Rahman ME, Fahal AH. Predictors of post-operative mycetoma recurrence using machine-learning algorithms: The mycetoma research center experience. PLoS Negl Trop Dis 2016;10(10):e0005007.
[Crossref] [Google Scholar] [PubMed]
- Mattioni S, Develoux M, Brun S, Martin A, Jaureguy F, Naggara N, et al. Management of mycetomas in France. Med Mal Infect 2013;43(7):286-94.
[Crossref] [Google Scholar] [PubMed]
- Chandler DJ, Bonifaz A, van de Sande WW. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol 2023;14:1165273.
[Crossref] [Google Scholar] [PubMed]
- Lim W, Eadie K, Konings M, Rijnders B, Fahal AH, Oliver JD, et al. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J Antimicrob Chemother 2020;75(4):936-41.
[Crossref] [Google Scholar] [PubMed]
- Nyuykonge B, Siddig EE, Mhmoud NA, Nyaoke BA, Zijlstra EE, Verbon A, et al. Epidemiological cut-off values for itraconazole and ravuconazole for Madurella mycetomatis, the most common causative agent of mycetoma. Mycoses 2022;65(12):1170-8.
[Crossref] [Google Scholar] [PubMed]
- Buil JB, Rijs AJ, Meis JF, Birch M, Law D, Melchers WJ, et al. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 2017;72(9):2548-52.
[Crossref] [Google Scholar] [PubMed]
- Lim W, Melse Y, Konings M, Phat Duong H, Eadie K, Laleu B, et al. Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma. PLoS Negl Trop Dis 2018;12(4):e0006437.
[Crossref] [Google Scholar] [PubMed]
- Lim W, Nyuykonge B, Eadie K, Konings M, Smeets J, Fahal A, et al. Screening the pandemic response box identified benzimidazole carbamates, olorofim and ravuconazole as promising drug candidates for the treatment of eumycetoma. PLoS Negl Trop Dis 2022;16(2):e0010159.
[Crossref] [Google Scholar] [PubMed]
- Nyuykonge B, Lim W, van Amelsvoort L, Bonifaz A, Fahal A, Badali H, et al. Eumycetoma causative agents are inhibited in vitro by luliconazole, lanoconazole and ravuconazole. Mycoses 2022;65(6):650-5.
[Crossref] [Google Scholar] [PubMed]
- Konings M, Eadie K, Lim W, Fahal AH, Mouton J, Tesse N, et al. The synthetic synergistic cinnamon oil CIN-102 is active against Madurella mycetomatis, the most common causative agent of mycetoma. PLoS Negl Trop Dis 2021;15(6):e0009488.
[Crossref] [Google Scholar] [PubMed]
- Vieira M, Pinheiro C, Fernandes R, Noronha JP, Prudêncio C. Antimicrobial activity of quinoxaline 1,4-dioxide with 2- and 3-substituted derivatives. Microbiol Res 2014;169(4):287-93.
[Crossref] [Google Scholar] [PubMed]
- Carta A, Paglietti G, Nikookar ME, Sanna P, Sechi L, Zanetti S. Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity. Eur J Med Chem 2002;37(5):355-66.
[Crossref] [Google Scholar] [PubMed]
- Waring MJ, Ben-Hadda T, Kotchevar AT, Ramdani A, Touzani R, Elkadiri S, et al. 2,3-Bifunctionalized quinoxalines: Synthesis, DNA interactions and evaluation of anticancer, anti-tuberculosis and antifungal activity. Molecules 2002;7(8):641-56.
- Jampilek J. Recent advances in design of potential quinoxaline anti-infectives. Curr Med Chem 2014;21(38):4347-73.
[Crossref] [Google Scholar] [PubMed]