- Corresponding Author:
- Neeta Shrivastava
Department of Pharmacognosy and Phytochemistry, B. V. Patel Pharmaceutical Education and Research Development (PERD) Centre, S. G. Highway, Thaltej, Ahmedabad - 380 054
E-mail: neetashrivastava_perd@yahoo.co.in
| Date of Submission | 5 March 2010 |
| Date of Revision | 6 December 2010 |
| Date of Acceptance | 5 February 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 111-115 |
Abstract
Physalis minima is an important medicinal plant of Indian System of Medicine. This plant is reported for its diuretic, laxative and antiinflammatory activities. However, the plant is not well scrutinized for its antimicrobial potential. The major chemical constituents reported from the plant are phenolics and alkaloids, which suggest that the plant may turn out to be a potent antiinfective agent. The aim of the study was to find out the antibacterial potential of mature berries of P. minima using streak plate, well diffusion, determination of minimum inhibitory concentration and bioautographic methods against a battery of Gram positive and Gram negative bacterial strains. Results of the study showed that methanol and chloroform extracts of P. minima exhibited potent inhibitory activity against all the bacterial strains tested. Minimum inhibitory concentration found out was 100 µg in both the extracts. Bioautography assay showed polar compounds present in the crude extract are responsible for the antimicrobial action.
Keywords
Bioautography, minimum inhibitory concentration, Physalis minima, thin layer chromatography
In recent years, multiple-drug resistance of pathogenic microorganisms has developed because of indiscriminate use of available antimicrobial drugs [1]. In addition, these antibiotics are associated with undesirable adverse effects like hypersensitivity, allergic reactions [2,3]. Natural products especially herbal medicines can offer an effective solution to these problems [4,5]. In the present study we report antibacterial potential of Physalis minima Linn (Solanaceae). P. minima is an annual herb found throughout India, Baluchistan, Afghanistan, Tropical Africa and Australia [6] and is reported as one of the important medicinal plants in Indian Traditional System of Medicines. The plant majorly contains phenolics, alkaloids, steroids and flavonoids [7]. The presence of phenolics and alkaloids in large amount suggest that the plants could be a potent source of antimicrobial and antineoplastic agents [8]. In this study we have evaluated crude methanol and chloroform extracts of fresh mature berries of the plant for antibacterial activity against a battery of Gram-positive and Gram-negative bacterial strains (eleven ATCC cultures).
All the chemicals and solvents used for the study were procured from Rankem, Mumbai, India. The extract was concentrated in rotary evaporator procured from Buchi R110, Germany. Fresh mature berries of the authenticated plants (authenticated at Mehasana Urban Bank Institute of Biosciences, Ganpat University, Kherva, Gujarat, India) were collected from its natural habitat in and around Ahmedabad, Gujarat, India. A voucher specimen of the plant (BVPPERD/H/Pm-041207) is preserved in the herbarium section of the Pharmacognosy and Phytochemistry Department.
Fresh mature berries (40 g) were ground in a mortar and pestle and extracted with methanol (50 ml×4) by intermittent warming. The extract was concentrated in a rotary evaporator followed by evaporation to dryness at 50° in a water bath and stored in amber colored storage vial at 4–5° until used for experiment [9]. Fresh mature berries (20 g) were hydrolyzed with 100 ml 2N hydrochloric acid under reflux condition for 5 h. The extract was filtered and the marc was washed with distilled water and was made alkaline (pH 9) using 10 % sodium carbonate. After drying the alkaline extract in hot air oven at 50° it was refluxed with chloroform (50 ml×4). After refluxing the extract was evaporated to dryness and stored in amber colored storage vial at 4–5° until used for experiment [9].
A battery of eleven American Type Culture Collection (ATCC) strains of bacteria were used for the study, they are: Bacillus cereus (ATCC 11778), Bacillus pumulis (ATCC 14884), Bacillus subtilis (ATCC 6633), Bordetella bronchiseptia (ATCC 4617), Enterococcus faecalis (ATCC 8043), Escherichia coli (ATCC 10536), Klebsiella pneumoniae (ATCC 10031), Micrococcus luteus (ATCC 9341), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 29737), Staphylococcus epidermis (ATCC 6538) [10].
For assay, the extracts were reconstituted in dimethylsulphoxide (DMSO) and required test concentrations were mixed in bacterial growth medium. After gelling the medium, the bacterial strains were streaked on to it to check the activity [11]. Ciprofloxacin (Gift from Cadila Pharmaceuticals Ltd., Ahmedabad, India) 4 mg/ml was used as positive control.
Bacterial isolates were mixed individually into the bacterial growth medium and poured aseptically into the sterilized petri-plates. After gelling 8 mm wells were bored in the medium using cork borer and different concentration of test solution and positive control were added in the wells. The plates were incubated at 37°, and zone of inhibition was observed after 24 h of incubation [11]. Minimum inhibitory concentration of methanol and chloroform extracts was carried out by broth assay [11].
Methanol and chloroform extracts were applied separately onto the precoated silica gel 60F254 TLC plates (uniform thickness: 0.2 mm; E. Merck Germany). The plates with methanol extracts were developed in two separate mobile phases, non-polar having composition of hexane: ethyl acetate: methanol (8:1.6:0.5), and polar with butanol:glacial acetic acid:water (4:1:0.5), while plates of chloroform extracts were developed in hexane:ethyl acetate:methanol (8:1.6:0.5). The plates were developed to a distance of 8 cm, dried at room temperature. The developed TLC plates were transferred into the sterile petri-plates and onto it, antibiotic assay medium inoculated with 0.1 ml bacterial culture was poured such that it formed a 5 mm thick layer of the medium on the plates. The petri-plates were incubated at 37° for 24 h. After 24 h, a solution of nitroblue tetrazolium (NBT 0.3 mg/ ml) was poured uniformly over the surface of the medium and the plates were further incubated for 4 to 5 h. Zone of inhibition observed as clear spot on the plates against dark background of dye [12].
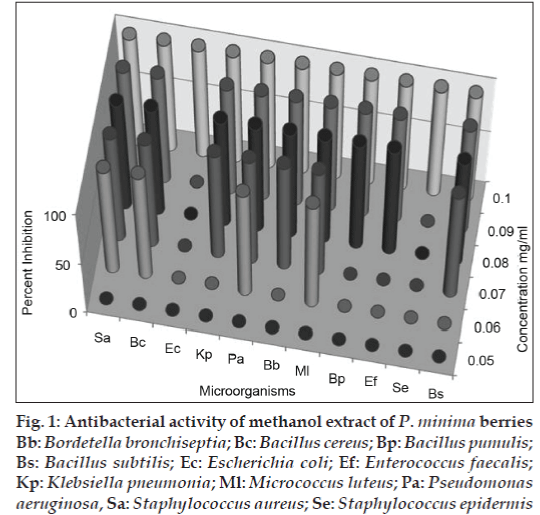
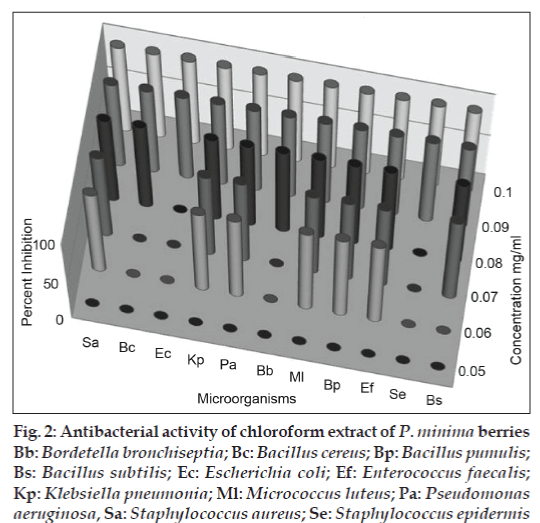
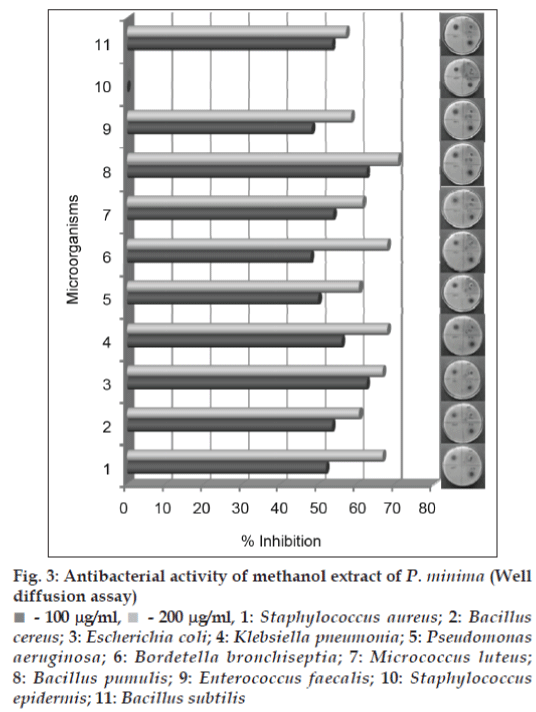
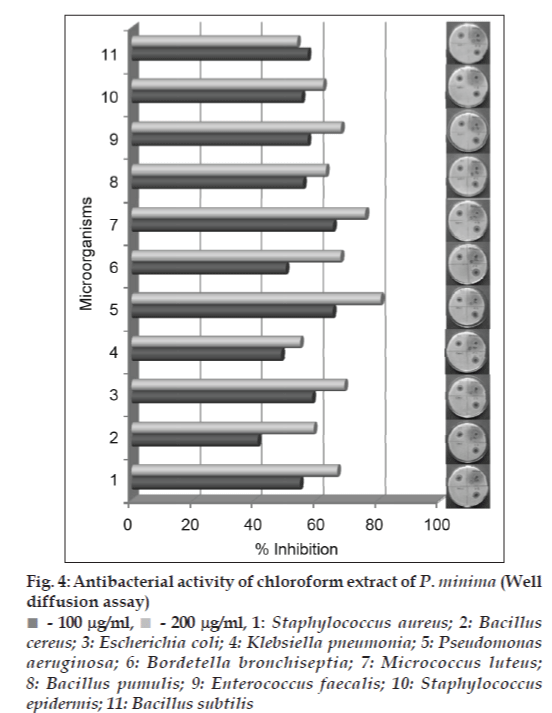
Both the methanol and chloroform extracts were screened in the concentration range of 0.05–2.0 mg/ ml, using streak plate method. The results obtained indicated complete inhibition of all the bacterial strains tested by both the extract at and above 100 μg/ml. For some of the bacterial strains, the inhibitory concentrations were observed as low as 0.06 mg/ml (figs. 1 and 2). For further confirmation of inhibitory action well diffusion assay was carried out at the concentration of 0.1 and 0.2 mg/ ml in both the extracts. In the study ciprofloxacin was used as positive control and the results were recorded as percent inhibition with respect to positive control. Both the methanol and chloroform extracts showed more than 50% inhibition in all the cultures tested, except S. epidermis, which did not show the inhibition in methanol extract (figs. 3 and 4). Minimum inhibitory concentration of both the extracts was determined by broth assay in the concentration range from 0.3-0.08 mg/ml. As observed in previous two experiments the MIC of methanol extracts was found out to be 100 μg/ml. However, chloroform extract being immiscible with aqueous medium could not be taken for broth assay.
Fig 1: Antibacterial activity of methanol extract of P. minima berries Bb: Bordetella bronchiseptia; Bc: Bacillus cereus; Bp: Bacillus pumulis; Bs: Bacillus subtilis; Ec: Escherichia coli; Ef: Enterococcus faecalis; Kp: Klebsiella pneumonia; Ml: Micrococcus luteus; Pa: Pseudomonas aeruginosa, Sa: Staphylococcus aureus; Se: Staphylococcus epidermis
Fig 2: Antibacterial activity of chloroform extract of P. minima berries Bb: Bordetella bronchiseptia; Bc: Bacillus cereus; Bp: Bacillus pumulis; Bs: Bacillus subtilis; Ec: Escherichia coli; Ef: Enterococcus faecalis; Kp: Klebsiella pneumonia; Ml: Micrococcus luteus; Pa: Pseudomonas aeruginosa, Sa: Staphylococcus aureus; Se: Staphylococcus epidermis
Fig 3: Antibacterial activity of methanol extract of P. minima (Well diffusion assay)
 - 100 mg/ml,
- 100 mg/ml,  - 200 mg/ml, 1: Staphylococcus aureus; 2: Bacillus cereus; 3: Escherichia coli; 4: Klebsiella pneumonia; 5: Pseudomonas aeruginosa; 6: Bordetella bronchiseptia; 7: Micrococcus luteus; 8: Bacillus pumulis; 9: Enterococcus faecalis; 10: Staphylococcus epidermis; 11: Bacillus subtilis
- 200 mg/ml, 1: Staphylococcus aureus; 2: Bacillus cereus; 3: Escherichia coli; 4: Klebsiella pneumonia; 5: Pseudomonas aeruginosa; 6: Bordetella bronchiseptia; 7: Micrococcus luteus; 8: Bacillus pumulis; 9: Enterococcus faecalis; 10: Staphylococcus epidermis; 11: Bacillus subtilis
Fig 4: Antibacterial activity of chloroform extract of P. minima (Well diffusion assay)
 - 100 mg/ml,
- 100 mg/ml,  - 200 mg/ml, 1: Staphylococcus aureus; 2: Bacillus cereus; 3: Escherichia coli; 4: Klebsiella pneumonia; 5: Pseudomonas aeruginosa; 6: Bordetella bronchiseptia; 7: Micrococcus luteus; 8: Bacillus pumulis; 9: Enterococcus faecalis; 10: Staphylococcus epidermis; 11: Bacillus subtilis
- 200 mg/ml, 1: Staphylococcus aureus; 2: Bacillus cereus; 3: Escherichia coli; 4: Klebsiella pneumonia; 5: Pseudomonas aeruginosa; 6: Bordetella bronchiseptia; 7: Micrococcus luteus; 8: Bacillus pumulis; 9: Enterococcus faecalis; 10: Staphylococcus epidermis; 11: Bacillus subtilis
The observed antimicrobial activity of both the crude extracts of P. minima could be result of a single active chemical constituent in the extracts or synergistic effect of many active chemical constituents of the plant present in the extract. Therefore, to identify bioactive fraction of both the crude extract, the extracts were fractionated using TLC method. As described in methodology, the extracts were applied on pre-coated silica plates and developed in polar and non-polar mobile phases to get maximum separation with good resolution of chemical constituents. The developed TLC plates were subjected to bioautography assay. The zone of inhibition obtained in the plates of methanol extract indicated that polar fractions of the crude methanol extract separated by TLC method are responsible for the antimicrobial activity because the zone of inhibition was observed at the application position on the TLC plate when it developed in non-polar mobile phase, while it was at the solvent front on the plate developed in polar mobile phase (fig. 5a). The zone of inhibition appeared as a blot instead of a discrete band indicating that 3-4 chemical constituents, which are polar in nature are active towards antibacterial activity. In case of chloroform extract, the zone of inhibition was obtained at two places in all the organisms indicating antibacterial activity of fraction separated at that location on the plate (fig. 5b). In bioautographic study, B. cereus and S. epidermis were not showing the zone of inhibition in methanol extract while growth of both of these organisms was inhibited in crude methanol extract. This indicates that the synergistic activity of various constituents present in crude extract was responsible for their growth inhibition.
Interest in higher plant extracts exhibiting antimicrobial activity has increased in recent years, and several reports in this area have been published [13-15]. The absolute alcohol, methanol, chloroform and petroleum ether extracts of leaves and callus of P. minima have been reported for antimicrobial activity with MIC values ranging up to 10 mg/ml [8]. Here we are reporting a detailed study on antimicrobial potential of the chloroform and methanol extract of fresh mature berries of the plant. The MIC values obtained in our study are much less (100 μg/ml, around 100 times less) than previously reported values on this plant indicating higher antimicrobial potential of fresh mature berries. In different reports, it has been stated that plants having MIC values up to 8 mg/ml can be explored as newer antibacterial agents [16,17]. The difference in the antimicrobial potential of the same plant in two reports could be the result of different geo-environmental conditions on gene expression responsible for secondary metabolite production and accumulation or the difference in plant parts. It is a well-known fact that in case of herbal medicines factors like geo-environmental conditions, plant parts, method of extraction and type of extracts influence the therapeutic potential.
Advances in molecular biology also suggest that epigenetic factor also influence in the gene expression of similar species. In this case, it is the plant part, type of extract as well as geo-environmental conditions, which are making difference. Further the steroidal glycosides are reported to be responsible for many biological activities in variety of plant species [18]. The presence of steroids and their glycosides have been reported in P. minima [19]. To extract these steroidal glycosides along with other polar chemical constituents the fresh mature berries of the plant were extracted with methanol while to extract sapogenins the berries were pretreated with 2N HCl to break the glycosidic linkage and extracted in chloroform. In the previous report, leaves of the plant were used as drug. The results in the present study showed that the extracts were active against both Gram-positive and Gram-negative bacteria presenting a broad spectrum activity. The exact mechanism of antibacterial effects from the plant can only be understood after further research. Results of bioautography assay also show that group of polar compounds are responsible for the activity; rather than single compound. The individual active compounds can only be known after their isolation, identification, and further studies in the area.
References
- Service RF. Antibiotics that resist resistance.Science 1995;270:724-7.
- Davies J. Inactivation of antibiotics and thedissemination of resistance genes. Science 1994;264:375-82.
- Enne VI, Livermore DM, Stephens P, Hall LM.Persistence of sulphanamide resistance in Escherichia coli in theUK despite national prescribing restriction. Lancet 2001;28:1325-8.
- Meurer-Grimes B, Mcbeth DL, Hallihan B, DelphS. Antimicrobial activity in medicinal plants of the Scrophulariaceae andAcanthaceae. Int J Pharmacogn1996;34:243-8.
- Rabe T, Van Staden J. Antibacterial activity ofSouth African plants used for medicinal purposes. J Ethnopharmacol1997;56:81-7.
- Kirtikar KR, Basu BD. Indian Medicinal PlantsVol. 3. Allahabad: Lalit Mohan Basu Publications; 1991.
- Khare CP. Indian Medicinal Plants Anillustrated Dictionary. New York: Springer Science Business Media, LLC;2007. p. 483.
- Shariff N, Sudarshana MS, Umesha S, HariprasadP. Antimicrobial activity of Rauvolfia tetraphylla and Physalisminima leaf and callus extracts. African J Biotech 2006;5:946-50.
- Houghton PJ, Raman A. Laboratory Handbook forthe Fractionation of Natural Extracts. London: Chapman and Hall; 1998.
- Indian Pharmacopoeia. Vol.2. (P-Z). New Delhi: The Controller of Publications; 1996. p. C103-4.
- Pelczar MJ. Microbiology. 5th ed. New Delhi:Tata McGraw-Hill Publishing Company Ltd.; 1993.
- Hamburger MO, Cordell GA. A directbioautographic TLC assay for compounds possessing antibacterial activity.J Nat Prod 1987;50:19-22.
- Farnsworth NR. Ethnopharmacology and drugdevelopment. Ciba Found Symp 1994;85:42-59.
- Benzi G, Ceci A. Herbal medicines in Europeanregulation. Phamacol Res 1997;35:355-62.
- Bagghi AK. Alternative medicine- Old wine in anew bottle. J Indian Med Assoc 2000;98:332-3.
- Garcia VM, Gonzalez A, Fuentes M, Aviles M,Rios MY, Zepeda G, etal. Antifungal activities of nine traditionalMexican medicinal plants. J Ethnopharmacol 2003;87:85-8.
- Garcia VM, Rojas G, ZepedaLG, Aviles M, Fuentes M, Herrera A, et al. Antifungal andantibacterial activity of four selected Mexicanmedicinal plants. PharmBiol 2006;44:297-300.
- Shrivastava N, Patel T. Clerodendrum and healthcare:An overview. Med Arom Plant Sci Biotech 2007;1:142-50.
- Sinha SC, Ali A, Bagchi A, Sahai M, Ray AB.Physalindicanols, new biogenetic precursors of C28-steroidal lactones from Physalis minima var. indica. Planta Med 1987;53:55-71.