- *Corresponding Author:
- M. J. Pati
Marathwada Mitra Mandal’s College of Pharmacy, Kalewadi (Thergaon), Pune - 411 017, India
E-mail: drmanoharpatil@yahoo.com
| Date of Submission | 19 July 2006 |
| Date of Revision | 28 May 2007 |
| Date of Acceptance | 16 December 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 827-831 |
Abstract
Protective effect of Tamarindus indica Linn (Caesalpiniaceae) was evaluated by intoxicating the rats with paracetamol (1 g/kg p.o.) for seven days. The aqueous extracts of different parts of Tamarindus indica such as fruits, leaves (350 mg/kg p.o.) and unroasted seeds (700 mg/kg p.o.) were administered for 9 days after the third dose of paracetamol. Biochemical estimations such as aspartate transaminase, alanine transaminase, alkaline phosphatase, total bilirubin and total protein were recorded on 4 th and 13 th day. Liver weight variation, thiopentone-induced sleeping time and histopathology were studied on 13 th day. Silymarin (100 mg/kg p.o.) was used as a standard. A significant hepatoregenerative effect was observed for the aqueous extracts of tamarind leaves, fruits and unroasted seeds (p<0.05) as judged from the parameters studied.
Keywords
Hepatoregenerative, paracetamol, Tamarindus indica Linn, silymarin
Tamarindus indica Linn (Caesalpiniaceae) is commonly known as tamarind, (Hindi: Imli) [1]. It grows as a large tree and is found all over India. T indica was found to be used in jaundice and other liver complaints in folk medicine [2,3]. Tamarind fruit contains high amount of ascorbic acid and β- carotene, which are proved to be potent antioxidant and hepatoprotective [4]. The aqueous extract of leaves contain ascorbic acid, β-carotene and are proved to be antilipoperoxidant, stops the peroxidation of tissue lipid and antihepatotoxic (in vitro) [5]. Pharmacological studies of the plant revealed that tamarind possess antibacterial, antidiabetic, antifungal, antiinflammatory, antimalarial and antioxidant activities [6]. Large doses of paracetamol will cause acute dose dependent necrosis in rats, mice and man [7]. Antioxidants can inhibit all the deleterious oxidative changes involved in paracetamolinduced toxicity [8].
Fruits and unroasted seeds were procured from Pune local market. The leaves were obtained from a tree near the college campus. The plant was authenticated by Botanical Survey of India, Pune, with voucher Specimen No. BP-1. The leaves were shade dried and crushed with hand and then extracted by decoction and filtered. Fruits were cleared for any dust or foreign material and then extracted by simple maceration. Unroasted seeds were pulverized to a coarse powder and macerated in water. All the extracts were concentrated under vacuum and were stored at 0-80 throughout the study. The yield of aqueous extract for fruits leaves and an unroasted seed was 74.06% w/w, 17.55% w/w and 6.44% w/w, respectively. Male Wistar rats weighing in the range of 150-200 g were used for the study [9]. They were obtained from National Toxicology Center, Pune. Animals were grouped in not more than five animals per cage. Animals were acclimatized to laboratory conditions for eight days before commencement of the experiment. They were allowed free access to standard dry pellet diet and water. Animals used in this study were treated and cared for in accordance with the guidelines recommended by the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) and the experimental protocol was approved by Institutional Animal Ethical Committee (IAEC/05-06/P-15).
Paracetamol was procured from Nulife Pharmaceuticals, Pune. Silymarin was purchased as Silybon-140 tablets (Micro Labs) and thiopentone sodium as Thiosol vial (Neon Labs) from the market. Enzymes like alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total protein and total bilirubin were assayed using standard kits from Nirmal Laboratories, India.
Animals were divided into six groups, each group comprising of five animals. All the animals except control group were intoxicated with paracetamol (1 g/ kg, p.o.) daily for 7 d. Group I received only vehicle i.e. water and served as control. Group II served as paracetamol control and received paracetamol (1 g/kg, p.o.)9 for first 7 d. Group III, silymarin, served as the positive control and received paracetamol (1 g/kg, p.o.) for first 7 d and silymarin (100 mg/kg, p.o.) [10] from 4th d to 12th d. Group IV (F), V (L) and VI (U) served as treated groups and received paracetamol (1 g/kg, p.o.) for first 7 d. The groups IV, V and VI received aqueous extract of fruits, leaves (350 mg/kg, p.o.) and unroasted seed (700 mg/kg, p.o.) respectively from 4th d to 12th. The dose of T. indica supplementation was selected by dose dependency study. This dose is the threshold dose and so the experiment was continued using the dose.
Blood was withdrawn by puncturing the retro-orbital plexus on d 4 and d 13. Serum was separated at 823×g. ALT, AST, ALP, total bilirubin and total protein levels were estimated using Reitman and Frankel method [11], Kind and King’s method [12], Evelyn and Malloy method [13] and Biuret method [14], respectively. On the 13th d, thiopentone sodium (37 mg/kg, i.p.) was injected to the animals and the sleeping time (min) was calculated as the interval lapsing between the loss and gain of the righting reflex [15]. The animals were then sacrificed with excess of light ether anesthesia. Liver was isolated, rinsed in water and weighed. Histopathology was performed by embedding the liver samples in separate paraffin blocks using conventional methods. Then the livers were cut into 3-5 μm thick section and stained in hematoxylin-eosin dye. Finally, these sections were mounted in diphenylxylene [15]. Data is expressed as mean ± SEM and is statistically assessed using ANOVA followed by Dunnett test. P<0.05 was considered as significant in all cases.
Tables 1-3 demonstrate the variations in the serum enzyme levels before and after drug treatment. On d 4, all the biochemical estimations such as ALT, AST, ALP, total bilirubin and total protein levels were found to be significantly increased (P<0.05) in all the paracetamol treated groups except control. On d 13 the serum levels of all the parameters of group IV, V and VI returned to control level. Sleeping time was prolonged in the paracetamol control group. The treated groups showed no significant increase (P<0.05) in the duration of sleep induced by thiopentone, when compared against paracetamol control group (Table 3).
| Groups | Biochemical parameters | |||||
|---|---|---|---|---|---|---|
| AST (Units/ml) | ALT (Units/ml) | |||||
| 4th d | 13th d | 4th d | 13th d | |||
| Control | 38.80 ± 3.86 | 040.51 ± 05.41 | 037.01 ± 03.08 | 037.60 ± 03.72 | ||
| Paracetamol control | 95.04 ± 8.84# | 145.20 ± 13.07# | 110.55 ± 12.31# | 174.56 ± 15.05# | ||
| Silymarin | 96.06 ± 9.41# | 059.60 ± 07.72** | 111.80 ± 11.02# | 065.60 ± 09.93** | ||
| F | 91.23 ± 6.31# | 070.81 ± 05.08** | 112.46 ± 12.22# | 089.60 ± 07.71** | ||
| L | 98.66 ± 8.32# | 064.40 ± 03.85** | 109.84 ± 10.21# | 078.41 ± 08.16** | ||
| U | 94.02 ± 9.34# | 100.21 ± 12.60** | 110.45 ± 10.67# | 122.40 ± 18.31** | ||
Table 1: Changes in serum ast and alt before and after treatment with t. Indica extracts.
| Groups | Biochemical parameters | ||||
|---|---|---|---|---|---|
| Total bilirubin (mg/dl) | ALP (KA Units/dl) | ||||
| 4th d | 13th d | 4th d | 13th d | ||
| Control | 0.421 ± 0.036 | 10.26 ± 1.43 | 10.4 ± 1.16 | 0.416 ± 0.026 | |
| Paracetamol control | 0.798 ± 0.072# | 34.69 ± 4.13# | 54.0 ± 3.16# | 1.544 ± 0.018# | |
| Silymarin | 0.788 ± 0.072# | 36.55 ± 3.22# | 14.8 ± 1.85** | 0.521 ± 0.008** | |
| F | 0.817 ± 0.080# | 40.31 ± 5.35# | 30.8 ± 1.93** | 0.740 ± 0.020** | |
| L | 0.850 ± 0.068# | 32.41 ± 6.14# | 20.8 ± 1.85** | 0.624 ± 0.011** | |
| U | 0.752 ± 0.062# | 36.64 ± 2.92# | 39.4 ± 2.48* | 1.096 ± 0.065** | |
Table 2: Changes in serum alp and total bilirubin before and after treatment with t. Indica extracts.
| Groups | Total protein (g/dl) | Sleeping time (min) | Liver weight (g/100 g of body weight) | |
|---|---|---|---|---|
| 4th d | 13th d | 13th d | 13th d | |
| Control | 8.02 ± 1.32 | 7.62 ± 0.73 | 019.66 ± 02.69 | 3.27 ± 0.61 |
| Paracetamol control | 5.16 ± 0.42# | 3.70 ± 0.35# | 246.64 ± 23.26# | 4.18 ± 0.72# |
| Silymarin | 5.21 ± 0.33# | 6.84 ± 0.71** | 040.54 ± 04.05** | 3.43 ± 0.14** |
| F | 5.36 ± 0.49# | 5.32 ± 0.46** | 059.60 ± 02.24** | 3.46 ± 0.65** |
| L | 5.25 ± 0.91# | 6.52 ± 0.55** | 052.59 ± 02.47** | 3.58 ± 0.68** |
| U | 5.15 ± 0.81# | 4.98 ± 0.40* | 112.80 ± 06.62** | 3.57 ± 0.54** |
Table 3: Changes in serum total protein levels and functional and morphological characteristics before and after treatment with t. Indica extracts.
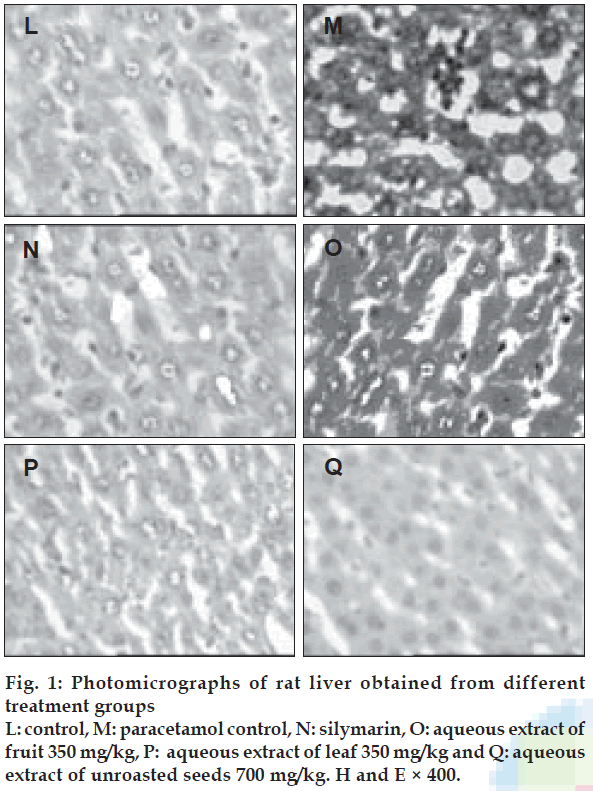
There was an increase in the weight of the liver in paracetamol control group (P<0.05), when compared with the control. The groups treated with T. indica extracts exhibited significantly lower liver weight (P<0.05), when compared with the paracetamol control group (Table 3). Table 4 and figs. 1 (L-Q) shows the histopathology of the livers of control, paracetamol control, silymarin treated, and T. indica extracts treated groups, respectively. Control group shows normal hepatic cells, paracetamol administration caused gross necrosis and hydropic lesions. Treatment with T. indica extracts reversed the hepatic lesions produced by paracetamol to a large extent.
| Microscopic observation | Control | Paracetamol control | Silymarin | F | L | U |
|---|---|---|---|---|---|---|
| Nuclear disintegration | - | +++ | - | - | + | - |
| Chromatolysis | - | ++ | - | - | - | - |
| Cytoplasmic vacuolation | - | ++ | - | + | - | + |
| Necrobiosis | - | + | - | - | - | - |
| Necrosis | - | +++ | - | - | - | - |
| Kuppfer cell hyperplasia | - | +++ | - | + | - | ++ |
| Portal inßammation | - | - | - | - | - | - |
| Sinusoidal dialation | - | ++ | - | - | - | - |
| Central venous dialation | - | + | - | - | - | - |
| Increased cytoplasmic eosinophilia | - | +++ | + | + | + | + |
Table 4: Histopathological changes in paracetamol-Induced liver injury in rats.
Paracetamol is known to produce acute liver damage if overdoses have been consumed. It is mainly metabolized in the liver to glucuronide and sulphate conjugates that are subsequently excreted. The hepatotoxicity of paracetamol has been attributed to the formation a highly reactive metabolite Nacetyl- P-benzoquinoneimine (NAPQI) by the hepatic cytochrome P-450. NAPQI is initially detoxified by conjugation with reduced glutathione (GSH) to form mercapturic acid. However, when the rate of NAPQI formation exceeds the rate of detoxification by GSH, it oxidizes tissue macromolecules such as lipid or thio (-SH) group of protein and alters the homeostasis of calcium after depleting GSH [16].
Assessment of liver damage is usually made by determination of serum enzyme levels of ALT, AST and ALP. Necrosis results in the release of these enzymes into circulation; therefore, it can be measured in serum. High levels of AST indicate liver damage, ALT catalyses the conversion of alanine to pyruvate and glutamate and is released in similar manner. Therefore, ALT is more specific to liver, and is thus a better parameter for detecting liver damage [17]. The results demonstrated that T. indica extracts caused significant decrease in serum ALT and AST levels. Serum ALP and total bilirubin levels are related to the function of hepatic cells. Increase in serum ALP is due to increased synthesis, in presence of increasing biliary pressure [18]. The results of the study indicated that the T. indica extracts significantly lowered the ALP and bilirubin level. Effective control of bilirubin and ALP activity points towards an early improvement in the secretary mechanism of hepatic cells.
It has been established that since barbiturates are metabolized exclusively in the liver, the sleeping time after a given dose is a measure of hepatic metabolism. If there is any pre-existing liver damage, in this case paracetamol-induced toxicity, the sleeping time after a given dose of thiopentone sodium will be prolonged because the amount of hypnotic broken down per unit time will be less [15]. The ability of T. indica extracts to reduce the prolongation of thiopentone-induced sleeping time in rats challenged with paracetamol is suggestive of the hepatoprotective potential of these extracts.
An increase in liver weight was observed. All the test groups showed significant reduction in the liver weight when compared with paracetamol control group. Histopathology of the liver samples revealed that the necrosis was reduced to few inflammatory cells in the rats treated with T. indica extracts. Cytoplasmic vacuolations and hydropic changes were less prominent. Inflammation of portal veins was also reduced. Thus the histopathological study shows reduction of degree of necrosis in the rats treated with T. indica extracts. It has been reported that T. indica contains flavonoids, ascorbic acid and β carotene [3]. A number of scientific reports indicated that flavonoids, ascorbic acid and β carotene have protective effect on liver due to their antioxidant properties [4,19]. Presence of those compounds in T. indica may be responsible for its protective effect on paracetamol-induced liver damage in rats. Based on the results of the present study, it can be concluded that the aqueous extracts of T. indica suppresses paracetamol-induced cell damage. Further investigations with isolated active principles of the plant may throw more light on the use of T. indica for hepatoprotective activity.
References
- Benson L. Plant classification. 2nded. London: Oxford and IBH Publishing Co; 1989.
- Nadkarni AM. Indian MateriaMedica. 3rded. Mumbai: Popular PrakashanPvt. Ltd; 1976.
- Ross IA. Medicinal plants of the world. chemical constituents, traditional and modern medicinal uses. 2nded. New Jersey: Humana Press; 2004.
- Olatunde FE, Oluwatosin AA, Godwin EO. Influence of chloramphenicol on rat hepatic microsomal components and biomarkers of oxidative stress: Protective role of antioxidants. PharmacolToxicol 2002;91:129-34.
- Joyeux M, Mortier F, Fleurentin J. Screening of antiradical, antilipoperoxidant and hepatoprotective effects of nine plant extracts used in caribbean folk medicine. Phytother Res 1995;9:228-30.
- Jha N, Jha A, Pandey I.Tamarindusindica: Tamarind: Imli.Phytopharm 2005;6:1-6.
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Davis DC, Brodie BB. Acetaminophen-induced hepatic necrosis I role of drug metabolism. J PharmacolExpTher 1973;187:185.
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine a Cytochrome p-450 mediated oxidation product of acetaminophen. ProcNatlAcadSci USA 1982;81:1327-31.
- Rao PG, Rao G, Ramnarayan K, Srinivasan KK. Effect of hepatogard on paracetamol-induced liver injury in male albino rats. Indian Drugs 1993;30:41-6.
- Shankar MB, Parikh JR, Geetha M, Mehta RS, Saluja AK. Hepatoprotective activity of a benzopyrone fromTephrosiapurpureaPers. J Nat Rem 2005;5:115-20.
- Reitman S, Frankel SA. Chlorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvate transaminases. Am J ClinPathol 1957;28:56-63.
- Kind PRN, King EJ. Estimation of plasma phosphatases by determination of hydrolyzed phenol with antipyrene. J ClinPathol 1954;7:322-30.
- Malloy HT, Evelyn KA. The determination of bilirubin with photometric colorimeter. J BiolChem 1949;4:481-90.
- Annino JS. Clinical chemistry, principle and procedure. 4th ed. Boston: Little brown and Company; 1976.
- Vishwakarma SL, Goyal RK. Hepatoprotective activity ofEnicostemmalittoralein CCl4- induced liver damage. J Nat Rem 2004;4:120-6.
- Gupta M, Mazumder UP, Sivakumar T, Gomathi P, Ramanathan SK. Antioxidant and hepatoprotective effect ofBauhinia racemosaagainst paracetamol and carbon tetrachloride-induced liver damage in rats. Iran J PharmacolTher 2004;3:12-20.
- Willianson EM, Okpako DT, Evans FJ. Selection, preparation and pharmacological evaluation of plant material. England: John Wiley; 1996.
- Moss DW, Butterworth PJ. Enzymology and medicine. London: Pitman Medical; 1974.
- Das KK, Dasgupta S. Influence of ascorbic acid on acid and alkaline phosphatase activities in some metabolically active tissues of aspirin treated rats. Indian J PhysiolPharmacol 1997;41:421-3.