- *Corresponding Author:
- Rajani Mathur
Department of Pharmacology, Delhi Institute of Pharmaceutical Sciences and Research, Pushp Vihar, New Delhi‑110 017, India
E‑mail: mathurajani@gmail.com
| Date of Submission | 17 July 2014 |
| Date of Revision | 21 January 2015 |
| Date of Acceptance | 07 August 2015 |
| Indian J Pharm Sci 2015;77(4):493-499 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The present study investigates the interaction of aqueous leaf extract of Psidium guajava with muscarinic, serotonergic and adrenergic receptor system using isolated rat ileum, gastric fundus and trachea, respectively. The concentration-dependent contractile response of aqueous leaf extract of Psidium guajava was parallel and rightward of standard agonists, ACh and 5-HT indicating agonistic activity on muscarinic and serotonergic receptor systems. The inhibition of aqueous leaf extract of Psidium guajava mediated contractions in presence of atropine (10-7 M) and ketanserin (10-6 M) confirmed the activity. Relaxant effect of PG (0.2 mg/ml) on carbachol induced pre-contracted rat tracheal chain indicated its agonistic action on adrenergic receptor system. Inhibition (P<0.05) of the action in the presence of propranolol (1 ng/ml) confirmed the activity. It may be concluded that PG possesses agonistic action on muscarinic, serotonergic and adrenergic receptor systems.
Keywords
Psidium guajava, serotonergic receptors, muscarinic receptors, adrenergic receptors, ileum, gastric fundus, tracheal chain
Herbs have always been an integral part of society, valued for both their culinary and medicinal properties. With extensive use of herbal medicine throughout the world, health practitioners should be vigilant when taking clinical histories and remain updated about the beneficial or harmful effects of these treatments. Thus continuing research is necessary to elucidate the pharmacological mechanism underlying the biological effect of herbal medicines and stimulate future pharmaceutical development of therapeutically beneficial herbal drugs [1]. Among the various herbal medicinal plants, Psidium guajava Linn. (Myrtaceae) is one of the important medicinal plant available in tropical and subtropical countries that is widely used as food and folk medicine around the world. Psidium guajava is well known for its antispasmodic, antimicrobial, antidiarrhoeal and antihyperglycemic properties [2‑5]. Pharmacological studies have also demonstrated its anticough [6], antiinflammatory [7] and antihypertensive [8] action.
However, inspite of its wide use in the treatment of various pathologies owing to its many useful pharmacological properties, the interaction of Psidium guajava with receptor systems has not been studied till now. It is well established that various physiological and pathological conditions are mediated by agonists and antagonists acting via muscarinic, serotonergic and adrenergic receptor systems [9‑11]. Therefore, it becomes imperative to study the interaction of aqueous leaf extract of Psidium guajava, (PG), with muscarinic, serotonergic and adrenergic receptor systems so as to provide pharmacological basis for its therapeutic use, and the present study addresses this lacuna.
Fresh leaves of Psidium guajava L. were collected, identified (NHCP/NBPGR/2010‑33), weighed, washed, dried under shade and powdered. The leaf powder was mixed with distilled water (1/10 w/v) and refluxed at 100° for 1 h. The extract was filtered, frozen (‑30º) and lyophilized (‑70º), under vacuum) and the yield of lyophilized powder, PG was calculated as 4.76% w/w with respect to leaf powder.
Wistar albino rats weighing 150‑250 g were selected for the study. They were housed under standard laboratory conditions of temperature (25±2°) and dark:light cycle (12 h each) and fed standard laboratory chow (Pranav Agro Industries Ltd, India) and drinking water. All experimental procedures were approved by Institutional Animal Ethical Committee (IAEC/2013/19) and conducted in accordance with the same.
Overnight fasted rats with free access to drinking water were euthanized under CO2. Following isolation, ileum was placed in oxygenated Tyrode solution (NaCl 137 mM, KCl 2.7 mM, CaCl2 1.8 mM, MgCl2 1 mM, NaHCO3 11.9 mM, NaH2PO4 0.4 mM and glucose; 5.55 mM) whereas gastric fundus and trachea were placed in oxygenated Krebs solution (NaCl 118 mM, KCl 4.7 mM, CaCl2 2.5 mM, MgSO4 1.2 mM, NaHCO3 25.0 mM, KH2PO4 1.2 mM and Glucose 5.55 mM). One end of the small segment (1 cm approx) of the tissue were mounted in organ bath (50 ml) maintained at 37° with aeration and the other end was tied to external isometric force transducer (Radnoti Single Channel Organ Bath, USA). Data was acquired using polyVIEW 16 (version 1.1) data acquisition system (Grass Technologies, USA) that had been previously calibrated as per the standard operating procedures provided by the manufacturer.
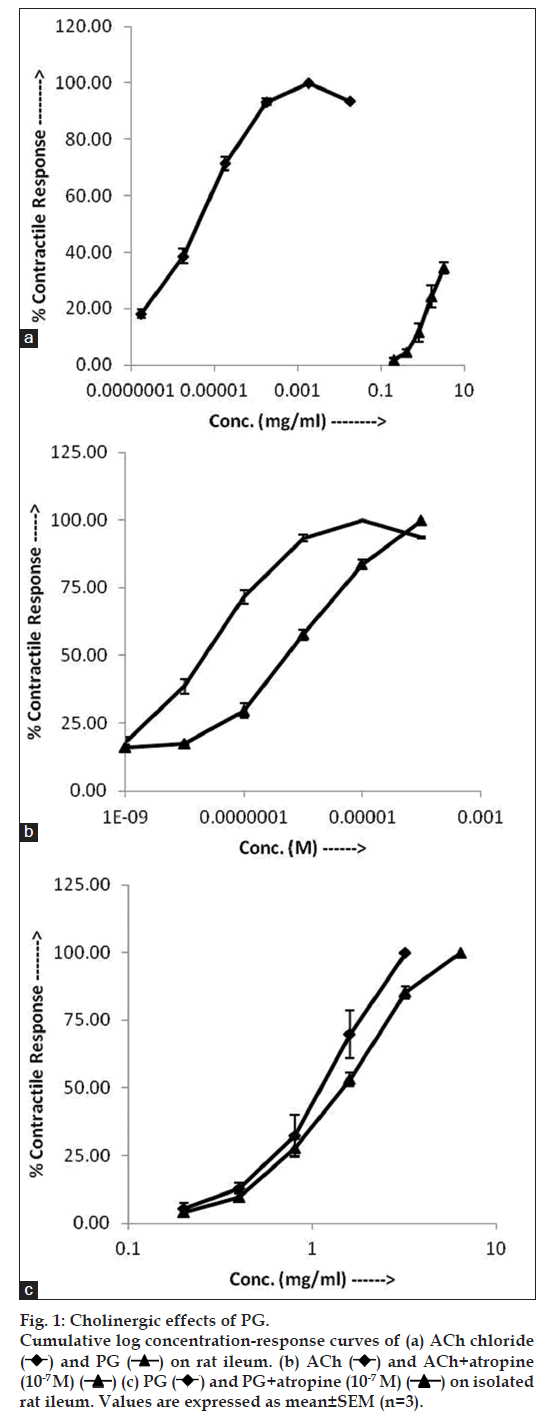
After mounting, the tissue was allowed to equilibrate for 30 min with repeated washing and the following concentration response curve (CRC) were recorded: a ACh (10‑9 to 10‑4 M); b. Ach (10‑9 to 10‑4 M)+atropine (10‑7 M); c. PG (0.2 to 3.2 mg/ml) and d. PG (0.2 to 3.2 mg/ml)+atropine (10‑7 M).
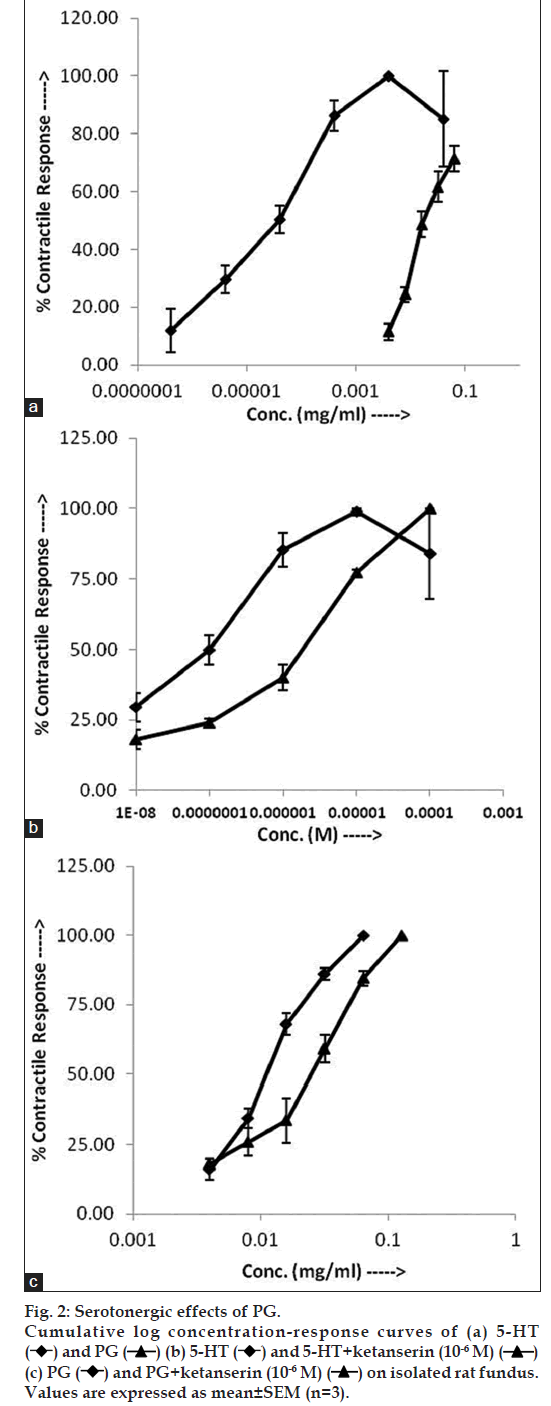
Gastric fundal strip were mounted and equilibrated and following CRC were recorded isometrically: a. 5‑HT (10‑9 to 10‑5 M); b. 5‑HT (10‑9 to 10‑5 M)+ketanserin (10‑6 M); c. PG (4 to 64 μg/ml) and d. PG (4 to 64 μg/ml)+ketanserin (10‑6 M).
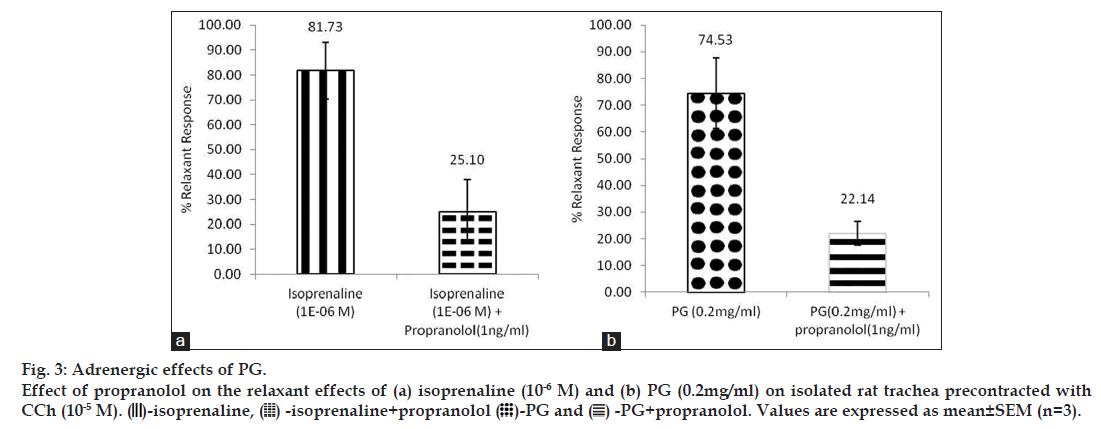
Each trachea was sectioned to give 2‑3 C‑shaped cartilaginous rings and tied to prepare tracheal chain as per method described [12]. Equilibrated tracheal chain was pre‑contracted with sub‑maximal concentration of carbachol (selected as 10‑5 M from 10‑9‑10‑4 M) and the relaxant effect of either standard isoprenaline (10‑6 M) or PG (0.2 mg/ml) was recorded. To confirm the response, the exercise was repeated in presence of standard antagonist, propranolol (1 ng/ml). All the data are expressed as mean±SEM (n=3). Student t‑test was used to compare the two groups. Significance was accepted at P<0.05.
Both ACh and PG produced concentration dependent contractions on ileum. The log CRC of PG was right ward in comparison to log CRC of ACh (fig. 1a) and the EC50 of PG (0.96±0.10 mg/ml) was significantly higher (P<0.01) than that of ACh (4.1±0.56× 10‑6 mg/ml). Moreover mean maximal response of PG (515.41±18.17 mg) was found significantly lower (P<0.05) than that of ACh (1500.38±37.02 mg) (fig. 1a). In the presence of atropine (10‑7 M), there was a right ward shift in the CRC of ACh (fig. 1b) and the EC50 was significantly (P<0.05) raised from 2.23±0.31×10‑8 to 2.92±0.24×10‑7 M. In the presence of atropine (10‑7 M), there was right ward shift in the CRC of PG (fig. 1c) and the EC50 was increased from 0.96±0.10 to 1.31±0.06 mg/ml.
5‑HT as well as PG induced concentration‑dependent contractions of gastric fundal strip. The log CRC of PG was on the right side of 5‑HT (fig. 2a) and the EC50 of PG (0.019±0.002 mg/ml) was significantly higher (P<0.05) than that of 5‑HT (2.86±0.57×10‑5 mg/ml). The maximal response of PG (515.41±18.17 mg) was found significantly lower (P<0.05) than that of 5‑HT (875.73±20.74 mg). In the presence of ketanserin (10‑6 M), there was a right ward shift in the CRC of 5‑HT (fig. 2b) and the EC50 of 5‑HT was significantly raised (P<0.05) from 1.17±0.07×10‑6 to 7.05±1.41×10‑8 M. In the presence of ketanserin (10‑6 M), right ward shift in the CRC of PG was recorded (fig. 2c) and the EC50 of PG was significantly increased (P<0.05) from 1.13±0.04×10‑2 to 2.01±0.21×10‑2 mg/ml.
Isoprenaline (10-6 M) exerted relaxant effects of 81.75% on rat tracheal chain precontracted with carbachol (10-5 M). In the presence of propranolol (1 ng/ml), the relaxant effect of isoprenaline (10-6 M) was reduced significantly (P<0.05) to 25.10% (fig. 3a). PG (0.2 mg/ml) produced relaxant effect of 74.74% on carbachol (10‑5 M) precontracted tracheal chain. The relaxant effect was reduced significantly (P<0.05) to 22.14% in the presence of propranolol (1 ng/ml) (fig. 3b).
Molecular‑cloning studies have revealed the existence of five molecularly distinct mammalian muscarinic acetylcholine receptor subtypes, M1–M5 [13] that can be subdivided into two major functional classes according to their G‑protein coupling preference, namely M1, M3 and M5 belong to Gq/G11 whereas the M2 and M4 belong to Gi/Go family [9,13]. Both M2 and M3 muscarinic receptors are expressed in smooth muscle of gastrointestinal tract and influence contraction. M3 receptors interact with Gq to trigger phosphoinositide hydrolysis, Ca2+ mobilization and a direct contractile response.
In contrast, M2 receptors interact with Gi and Go to inhibit adenylyl cyclase and Ca2+‑activated K+ channels to potentiate a Ca2+‑dependent, nonselective cation conductance. Ultimately, these mechanisms lead to the prediction that the influence of the M2 receptor on contraction should be conditional upon mobilization of Ca2+ by another receptor such as the M3. Mathematical modelling studies of these mechanisms show that the competitive antagonism of a muscarinic response mediated through activation of both M2 and M3 receptors should resemble the profile of the directly acting receptor (i.e., the M3) and not that of the conditionally acting receptor i.e., the M2 [14]. Normal function of digestive system depends in part, on normal intestinal movements. These coordinated movements are regulated by complex mechanisms involving different receptors within the gut wall and their interaction with various substances with inhibitory or excitatory effects. These receptors maintain gut reflex activity and peristalsis even when disconnected from the CNS [15]. Thus study of muscarinic and serotonergic receptors on isolated ileum and gastric fundus and various substances which affect their function is one of the leading topics in the gastroenterology research.
In our study, the affinity of aqueous leaves extracts of Psidium guajava for muscarinic receptor system was examined using isolated rat ileum. The result demonstrated that ACh as well as PG increased the contractile response in rat ileum. The contractile response induced by PG was lesser in potency as well as efficacy than ACh as the EC50 value of PG was found significantly higher than ACh and maximal response significantly lesser than ACh. The EC50 values of ACh as well as PG were found to be higher in the presence of muscarinic antagonist atropine as compared to those of ACh and PG alone, respectively. These finding suggest that the contractile effect of Psidium guajava is mediated partly through muscarinic receptors. However, involvement of mechanism other than muscarinic need to be confirmed using other tissue model.
Serotonin (5‑hydroxytryptamine, 5‑HT) produces its effects through a variety of membrane‑bound receptors. 5‑HT and its receptors are found both in the central and peripheral nervous system as well as in a number of non‑neuronal tissues in the gut, cardiovascular system and blood. In evolutionary terms, 5‑HT is one of the oldest neurotransmitters and has been implicated in the aetiology of numerous disease states, including depression, anxiety, social phobia, schizophrenia, and obsessive compulsive and panic disorders; in addition to migraine, hypertension, pulmonary hypertension, eating disorders, vomiting and, more recently, irritable bowel syndrome. With the exception of the 5‑HT3 receptor, which is a ligand‑gated ion channel, 5‑HT receptors belong to the G‑protein‑coupled receptor superfamily and, with at least 14 distinct members, represent one of the most complex families of neurotransmitter receptors [16].
The serotonergic affinty of PG extract was examined and the results show that, 5‑HT as well as PG increased the contractile response in rat fundus. The contractile response induced by PG was lesser in potency as well as efficacy than 5‑HT. The EC50 values of 5‑HT as well as PG were found to be significantly higher in the presence of serotonergic antagonist ketanserin as compared to those of 5‑HT and PG alone, respectively. It has been reported that ketanserin antagonizes high‑affinity responses of 5‑HT2B receptor agonist on rat gastric fundus [17]. This finding implies that PG extracts mediated the contractile effect on rat gastric fundus mainly due to the serotonergic properties of PG. The rat gastric fundus possesses contractile 5‑HT2B receptors and relaxant 5‑HT2A and 5‑HT4 receptors but is enriched with the 5‑HT2B receptor [18,19]. Unlike other members of the 5‑HT2 receptor family, the 5‑HT2B receptor in the rat stomach fundus is not coupled to phosphatidylinositol (PI) hydrolysis. It has been reported that the 5‑HT2B contractile receptor in the rat stomach fundus is coupled to calcium influx through voltage‑dependent calcium channels, intracellular calcium release, and activation of PKC. These actions may reflect a novel coupling mechanism unrelated to increases in PI hydrolysis [19]. So it seems likely that PG exerted its contratile response on gastric fundus via 5‑HT2B by coupling to calcium influx through voltage‑dependent calcium channels, intracellular calcium release, and activation of PKC.
The adrenergic system plays an important role in regulating body homeostasis in health and disease. A large number of drugs have been developed to modulate adrenergic signaling. These drugs interact with a group of adrenergic receptors, which belong to the family of G‑protein‑coupled receptors [20].
Several pathways are known to involve in the relaxation of isolated trachea including β2‑agonism, H2‑ antagonism, NO induced relaxation and prostonoids‑induced relaxation [21‑24]. The exact cause of relaxant effect of β2‑agonists in carbachol‑contracted rat tracheal muscle is not yet clear. However, it has been reported activation of β‑adrenoceptors produces relaxation of smooth muscle tissue or a reduction in the amplitude of contractions evoked by various stimulants, and according to current views, relaxation of smooth muscle occurs when there is a significant reduction in the cytoplasmic free Ca2+ concentration [25] and/or when there is an inhibition of actin‑myosin interaction due to phosphorylation of myosin light chain kinase [26]. So these mechanisms, may involve in β‑agonism on pre‑contracted trachea with carbachol that were observed in our study.
Furthermore, the main active constituent in the Psidium guajava is reported to be quercetin [27]. Preliminary phytochemical analysis of PG confirmed the presence of alkaloids, flavonoids, terpenoids, steroids, tannins and carbohydrates. Using standardized protocols of RP‑HPLC, the concentration of Quercetin in PG was found to be 8.005 μg/ml (data not shown here). Earlier, it is also known that quercetin inhibits rat tracheal contractility through a presynaptic involving nitric oxide [28]. Psidium guajava also known to contain sesquiterpenes as one of the phytocostituent [29]. Sesquiterpenes stimulate SGC which through the activation of cGMP and PKG pathway stimulate extraction of K+ ions thereby reduce the intrinsic Ca2+ ions, leading to relaxation of smooth muscles [30]. On the basis of these finding, it seems that Psidium guajava leaf extract exerts its relaxant effect on tracheal smooth muscle involving more than one mechanism that may due to the presence of several phytoconstituent in the PG extract.
It is known that β2‑agonists are used for treatment of asthma [31]. Previous findings have shown that treatment with quercetin inhibits the release of histamine and proinflammatory mediators (TNF‑α, IL‑1β, IL‑6 and IL‑8) from mast cells stimulated with IgE, likely due to inhibition of NF‑kB and mitogen‑activated protein kinase [32]. Thus, these findings pave the way for the PG in the treatment asthma. Hence from the present study it can be concluded that PG possesses agonistic action on serotonergic, adrenergic and muscarinic receptor systems.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch Intern Med 1998;158:2225‑34.
- Lozoya X, Reyes‑Morales H, Chávez‑Soto MA, Martínez‑García Mdel C, Soto‑González Y, Doubova SV. Intestinal anti‑spasmodic effect of a phytodrug of Psidium guajava folia in the treatment of acute diarrheic disease. J Ethnopharmacol 2002;83:19‑24.
- Chah KF, Eze CA, Emuelosi CE, Esimone CO. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J Ethnopharmacol 2006;104:164‑7.
- Ojewole JA, Awe EO, Chiwororo WD. Antidiarrhoeal activity of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rodents.J Smooth Muscle Res 2008;44:195‑207.
- Cheng JT, Yang RS. Hypoglycemic effect of guava juice in mice and human subjects. Am J Chin Med 1983;11:74‑6.
- Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, Saraya ML, et al. Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. J Ethnopharmacol 1999;67:203‑12.
- Shaheen HM, Ali BH, Alqarawi AA, Bashir AK. Effect of Psidium guajava leaves on some aspects of the central nervous system in mice.Phytother Res 2000;14:107‑11.
- Ojewole JA. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find Exp Clin Pharmacol 2005;27:689‑95.
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: Mutant mice provide new insights for drug development. Nat Rev Drug Discov 2007;6:721‑33.
- Houston DS, Vanhoutte PM. Serotonin and the vascular system. Role in health and disease, and implications for therapy. Drugs 1986;31:149‑63.
- McGraw DW, Forbes SL, Kramer LA, Witte DP, Fortner CN, Paul RJ, et al. Transgenic overexpression of beta(2)‑adrenergic receptors in airway smooth muscle alters myocyte function and ablates bronchial hyperreactivity. J Biol Chem 1999;274:32241‑7.
- Kulkarni SK. Handbook of Experimental Pharmacology. New Delhi: Vallabh Prakashan; 2005.
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998;50:279‑90.
- Ehlert FJ. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci 2003;74:355‑66.
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 2007;19 1 Suppl: 1‑19.
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5‑HT receptors. Pharmacol Biochem Behav 2002;71:533‑54.
- Béjar E, Malone MH. Inhibitory effect of 5‑hydroxytryptamine on rat stomach fundus: Mediated indirectly by activation of noradrenaline release. J Pharm Pharmacol 1995;47:637‑42.
- Komada T, Yano S. Pharmacological characterization of 5‑hydroxytryptamine‑receptor subtypes in circular muscle from the rat stomach. Biol Pharm Bull 2007;30:508‑13.
- Cox DA, Cohen ML. 5‑HT2B receptor signaling in the rat stomach fundus: Dependence on calcium influx, calcium release and protein kinase C. Behav Brain Res 1996;73:289‑92.
- Pierce KL, Premont RT, Lefkowitz RJ. Seven‑transmembrane receptors. Nat Rev Mol Cell Biol 2002;3:639‑50.
- de Vries B, Meurs H, Roffel AF, Elzinga CR, Hoiting BH, de Vries MM, et al. Beta‑agonist‑induced constitutive beta(2)‑adrenergic receptor activity in bovine tracheal smooth muscle. Br J Pharmacol 2000;131:915‑20.
- Drazen JM, Venugopalan CS, Schneider MW. Alteration of histamine response by H2‑receptor antagonism in the Guinea pig. J Appl Physiol Respir Environ Exerc Physiol 1980;48:613‑8.
- Hwang TL, Wu CC, Teng CM. YC‑1 potentiates nitric oxide‑induced relaxation in Guinea‑pig trachea. Br J Pharmacol 1999;128:577‑84.
- Sipahi EY, Türker RK, Ercan ZS. Nitric oxide and prostanoid‑dependent relaxation induced by angiotensin II in the isolated precontracted mouse tracheal muscle and the role of potassium channels. Pharmacol Res 2000;42:69‑74.
- Kuriyama H, Ito Y, Suzuki H, Kitamura K, Itoh T. Factors modifying contraction‑relaxation cycle in vascular smooth muscles. Am J Physiol 1982;243:H641‑62.
- Adelstein RS, Conti MA, Hathaway DR, Klee CB. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3’: 5’‑monophosphate‑dependent protein kinase. J Biol Chem 1978;253:8347‑50.
- Seshadri TR, Vasistha K. Polyphenols of the leaves of Psidium guajava: Quercetin, guaijiaverin, leucocyanidin and amritoside. Phytochemistry 1965;4:989‑92.
- Capasso R, Aviello G, Romano B, Atorino G, Pagano E, Borrelli F. Inhibitory effect of quercetin on rat trachea contractility in vitro. J Pharm Pharmacol 2009;61:115‑9.
- Somchit MN, Sulaiman MR, Ahmad Z, Israf DA, Hosni H. Nonopioid antinociceptive effect of Psidium guajava leaves extract. J Nat Remedy 2004;4:174‑8.
- Liu B, Yang J, Wen Q, Li Y. Isoliquiritigenin, a flavonoid from licorice, relaxes Guinea‑pig tracheal smooth muscle in vitro and in vivo: Role of cGMP/PKG pathway. Eur J Pharmacol 2008;587:257‑66.
- Mukherjee AB, Zhang Z. Allergic asthma: Influence of genetic and environmental factors. J Biol Chem 2011;286:32883‑9.
- Rigolin FL, de Freitas AC, Martins TM, Paula RA. Quercetin: A flavonoid with the potential to treat asthma. Braz J Pharm Sci 2012;48:589‑99.