- *Corresponding Author:
- B. Lakshmi Narayanan
Department of Pharmacy, Annamalai University, Annamalainagar, Chidambaram-608 002, India
E-mail: blnrxpharma@gmail.com
| Date of Submission | 28 July 2016 |
| Date of Revision | 10 January 2017 |
| Date of Acceptance | 26 April 2017 |
| Indian J Pharm Sci 2017;79(3):477-482 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
An important medicinal plant, Polygonum barbatum was undertaken for gas chromatography-mass spectrometry analysis and high performance thin layer chromatography finger print profile to investigate the chemical constituent present in the hydroalcoholic extract of Polygonum barbatum leaves. Gas chromatography-mass spectrometry analysis shown that twenty two compounds were identified and named from the extract. Among these constituents, terpenoids are the major constituents present in this extract. Other major constituents were present in the extracts were carbohydrate and fatty acids. High performance thin layer chromatography fingerprint analysis of hydroalcoholic extract of P. barbatum was carried out by using ethyl acetate-hexane (7:3 v/v) as a mobile phase. From the high performance thin layer chromatography analysis, peak numbers 4, 5 and 6 shown highest % of peak area of 29.79, 21.42 and 10.67, respectively at 366 nm.
Keywords
Polygonum barbatum linn, hydroalcoholic extract, GC-MS analysis, HPTLC fingerprint
Polygonum barbatum belongs to the family Polygonacaea is one of the most important herbal used in the traditional medicine. The genus Polygonum is comprised of nearly 300 species, which are widely distributed around the world. The genus primarily grows in northern temperate regions and Asian countries. They vary widely from prostrate herbaceous annual plants under 5 cm high to erect herbaceous perennial plants growing up to 3-4 m tall to perennial woody vines growing up to 20-30 m high in trees. Several are aquatic, growing as floating plants in ponds. The smooth-edged leaves range from 1-30 cm long and vary in shape between species from narrow lanceolate to oval, broad triangular, heart-shaped, or arrowhead forms. The stems are often reddish or red-speckled. The small flowers are, pink, white, or greenish, forming in summer in dense clusters from the leaf joints or stem apices [1].
Various secondary metabolites like tannins [1], proanthocyanidins [2], flavonoids [3,4], anthraquinones [5], stilbenoids [6], resveratrol [7], lignans [8], phenylpropanoids [9], triterpenoids [10], coumarins and neoflavonoids [11], sesquiterpenoids [12], anthraquinones [13], phenylpropanoids [14], proteins, amino acids and carbohydrates [15] have been reported from species of genus Polygonum.
Among the various species of genus Polygonum, biological activities such as antiinflammatory activity of P. glabrum [16], anticancer activity of P. hydropiper L [17], antihypertensive effect of P. perfoliatum [18], myocardial protective action of P. multiflorum [19], antiviral activity of P. punctutam [20], antiallergic effect of P. tinctorium [21], analgesic, antiinflammatory and CNS depressant activities of sesquiterpenes and a flavonoid glycoside from P. viscosum [22], antioxidant activity of P. paleaceum [23] and antiulcer activity of P. barbatum [24] were reported. Review of literature shown that there was limited number analytical studies carried out on the species of P. barbatum.
The present communication deals with the gas chromatography mass spectrometry (GC-MS) and high performance thin layer chromatography (HPTLC) fingerprinting analysis of hydroalcoholic extract of P. barbatum (HAPB) leaves. P. barbatum leaves were collected from an Ukkadam area in Coimbatore, Tamil Nadu, India. It was identified and authenticated (FRL/ CRJ/RDR No. 04/2014-15) at the Foundation for Revitalisation of Local Health Traditions (FRLHT), Bangalore, Karnataka, India. The plant materials (leaves) were cleaned, shade dried and powdered. The powdered leaves of the plant (100 g) was transferred into stoppered flask and treated with ethanol (70 % v/v) until the powder was fully immersed. The flask was shaken every hour for the first 6 h and then it was kept aside and shaken after 24 h. This process was repeated for 2 d and then the extract was filtered. The extract was collected and evaporated to dryness by vacuum. The final residue thus obtained was then subjected to GCMS and HPTLC analysis. Preliminary phytochemical screening of HAPB was carried out by the standard methods and it was reported [24].
The HPTLC fingerprint profile was determined for HAPB by the method of Harborne [25] and Wagner et al. [26]. About 1.0 g of the extract was dissolved in ethanol 70% and was taken for analysis. HAPB was spot (2 μl) as bands with the help of the auto sampler fitted with a 100 μl Hamilton syringe on a 10×10 cm silica gel precoated 60F-254 aluminium HPTLC plates of thickness 0.2 mm was used. Different combination of solvent systems like toluene:ethyl acetate, benzene:ethyl acetate, ethyl acetate:ethanol, chloroform:ethanol, chloroform:methanol, chloroform:water, ethyl acetate:benzene, ethyl acetate:hexane with various proportion were tried to obtain an excellent separation and sharp peaks for analysis. The satisfactory resolution of the separation of compounds presented in HAPB was obtained in ethyl acetate: hexane (7:3 v/v) solvent system. After the application of sample, the plate was developed in twin trough glass chamber 10×10 to a distance of 8 cm. Twin trough glass chamber was previously saturated with the solvent system by 30 min. The developed plate was air dried and scanned at 366 nm using Camag scanner 3 with WINCATS software. The plate was photographed at 366 nm using Camag Reprostar 3. The retention factor (Rf) value of each compound was separated on plate and % peak area of each band was recorded.
The GC-MS analysis of HAPB was performed using a GC-MS equipment GC Clarus 500 Perkin Elmer. Experimental conditions of GC-MS system were as follows: Elite-5MS (5% diphenyl/95% dimethyl poly siloxane), dimention: 30×0.25 mm×0.25 μm df was used and flow rate of mobile phase (carrier gas: He) was set at 1.0 ml/min and an injection volume of 2 μl was employed (split ratio of 10:1 injector temperature 250°). The oven temperature was programmed from 110° (isothermal for 2 min) with an increase of 10°/ min up to 200°, then 5°/min up to 280° and ending with a 9 min isothermal at 280°. Mass spectra were taken at 70 eV, scan interval of 0.5 s and fragments from 45 to 450 Da. Source and inlet line temperature was 200° and the mass spectra detected at 36 min.
The GC-MS spectrum of HAPB was interpreted by using the database of National Institute Standard and Technology (NIST). The unknown individual constituents were identified based on direct comparison of the retention time (RT) and their mass spectra of known compounds stored in the NIST spectral database library. The RT, name, molecular formula, molecular weight and structure of the unknown compound were ascertained.
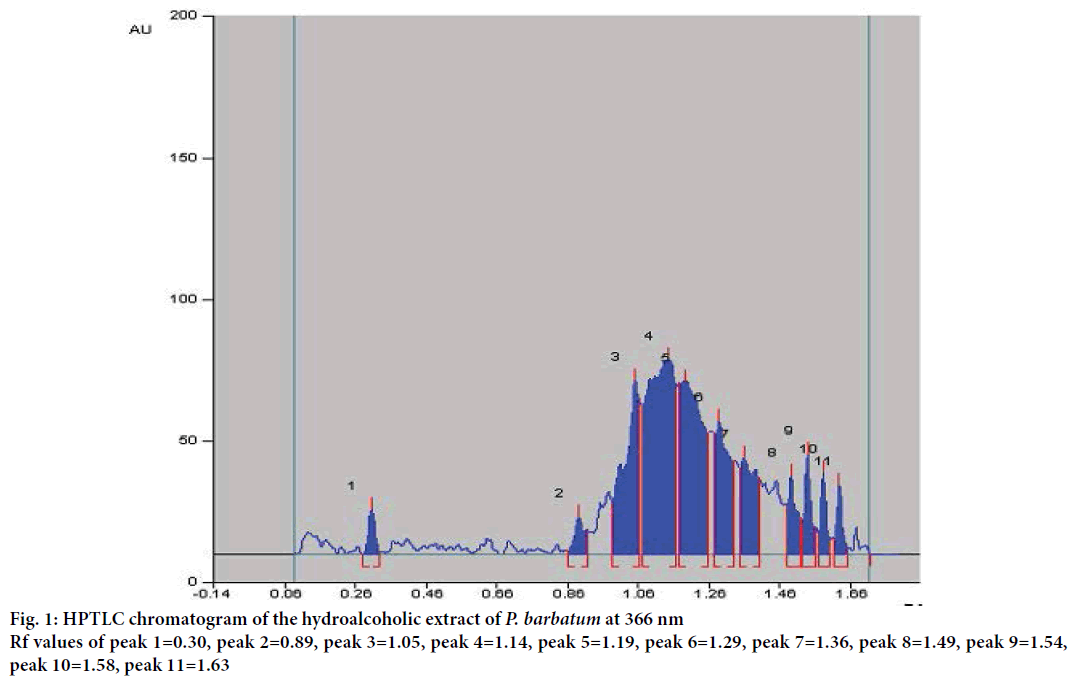
HPTLC fingerprint profile of HAPB was carried out by using ethyl acetate: hexane (7:3 v/v) solvent system to confirm the presence of various phytoconstituents in the extract. HPTLC chromatogram showed a total of 11 peaks at different Rf values and peak area at 366 nm was obtained (Figure 1, Table 1).
| Wavelength | Peaks | Rf values | %Peak area |
|---|---|---|---|
| 366 nm | 1 | 0.30 | 1.60 |
| 366 nm | 2 | 0.89 | 2.13 |
| 366 nm | 3 | 1.05 | 14.62 |
| 366 nm | 4 | 1.14 | 29.79 |
| 366 nm | 5 | 1.19 | 21.42 |
| 366 nm | 6 | 1.29 | 10.67 |
| 366 nm | 7 | 1.36 | 8.20 |
| 366 nm | 8 | 1.49 | 3.55 |
| 366 nm | 9 | 1.54 | 3.43 |
| 366 nm | 10 | 1.58 | 2.46 |
| 366 nm | 11 | 1.63 | 2.13 |
Table 1: HPTLC fingerprint profile of hydroalcoholic extract of Polygonum barbatum linn
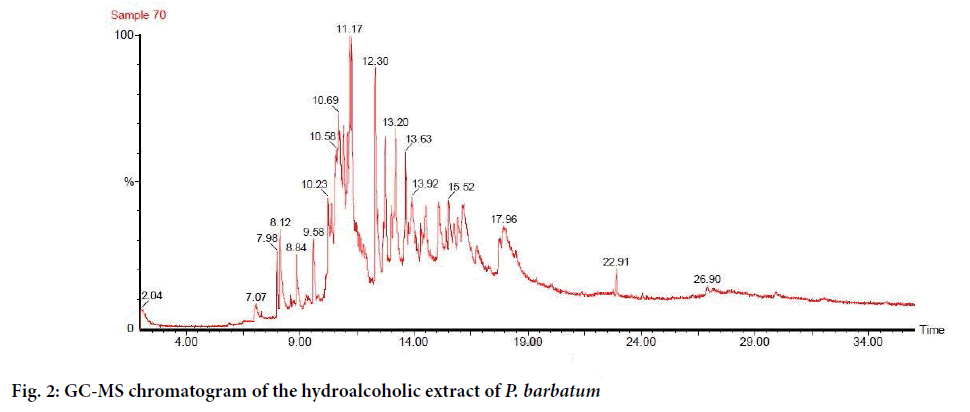
The results pertaining to GC-MS analysis lead to the identification of a number of compounds from gas chromatography fractions of the HAPB. They were identified through mass spectrum attached with GC. The GC-MS analysis of the HAPB was reported in Table 2.
| RT | Name of the Compound | Molecular formula | Molecular weight | Peak area (%) | Nature of the compounds |
|---|---|---|---|---|---|
| 7.07 | 7-Octene-1,2-diol | C8H16O2 | 144 | 1.01 | Mono terpene diol |
| 7.98 | 3,7-dimethyl-6-Octen-1-ol | C10H20O | 156 | 1.42 | Alcoholic compound |
| 8.12 | 1,4a,5,6,7,8,9,9a- octahydro-4a-methyl-, trans-2H-benzocyclohepten-2-one | C12H16O | 178 | 2.78 | Ketone |
| 8.84 | 4,5,5a,6,6a,6b- hexahydro-4,4,6b-trimethyl-2-(1-methylethenyl)- 2H-cyclopropa[g]benzofuran | C15H22O | 218 | 1.55 | Terpenoid |
| 9.58 | 1-methyl-4-(2- methyloxiranyl)-7-oxabicyclo[4.1.0]heptane | C10H16O2 | 168 | 1.50 | Terpenoid |
| 10.23 | 3,3,5-trimethyl-cyclohexene | C9H16 | 124 | 3.80 | Terpenoid |
| 10.69 | D-Mannose | C6H12O6 | 180 | 18.32 | Carbohydrate |
| 10.91 | Methyl ester of 2'-hexyl-[1,1'-Bicyclopropyl]-2-octanoic acid | C21H38O2 | 322 | 6.55 | Fatty acid |
| 11.09 | Longipinene epoxide | C15H24O | 220 | 6.21 | Terpenoid |
| 11.17 | Limonene-1,2-epoxide(fr.1) | C10H16O | 152 | 6.56 | Terpenoid |
| 12.30 | Aristolene epoxide | C15H24O | 220 | 5.72 | Terpenoid |
| 12.74 | 4,6-diisopropylidene-8,8-dimethyl-bicyclo[5.1.0]octan-2-one | C16H24O | 232 | 2.86 | Ketone |
| 13.20 | Aromadendrene oxide-(2) | C15H24O | 220 | 5.50 | Terpenoid |
| 13.63 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280 | 3.23 | Fatty acid |
| 13.92 | Methyl ester of2,5-octadecadiynoic acid | C19H30O2 | 290 | 4.10 | Fatty acid ester |
| 14.52 | trans-Z-à-Bisabolene epoxide | C15H24O | 220 | 2.86 | Terpenoid |
| 15.10 | cis-Z-à-bisabolene epoxide | C15H24O | 220 | 4.43 | Terpenoid |
| 15.52 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa- cyclopropa[a]inden-6-one | C13H20O2 | 208 | 3.70 | Terpenes |
| 16.19 | octahydro-1,4,9,9- tetramethyl-1H-3a,7-methanoazulene | C15H26 | 206 | 7.71 | Sesquiterpenes |
| 17.96 | Alloaromadendrene oxide-(1) | C15H24O | 220 | 8.75 | Sesquiterpenoid |
| 22.91 | 3,7,11-trimethyl-, (Z,E)-2,6,10-dodecatrien-1-ol | C15H26O | 222 | 0.61 | Fatty alcohol |
| 26.90 | Phenylmethyl ester 9-Octadecenoic acid | C25H40O2 | 372 | 0.83 | Fatty acid ester |
Table 2: Phytoconstituents in the hydroalcoholic extract of Polygonum barbatum by using GC-MS analysis
The results revealed that the presence of 22 different phytoconstituents viz., D-mannose (18.32%), alloaromadendrene oxide-(1) (8.75%), 1H-3a,7-methanoazulene, octahydro-1,4,9,9 tetramethyl-(7.71%), limonene-1,2-epoxide(fr.1) (6.56%), [1,1'-bicyclopropyl]-2-octanoic acid, 2'-hexyl-, methyl ester (6.55%), longipinene epoxide (6.21%), aristolene epoxide (5.72%), aromadendrene oxide-(2) (5.50%), cis-Z-à-bisabolene epoxide (4.43%), 2,5-octadecadiynoic acid, methyl ester (4.10%), cyclohexene, 3,3,5-trimethyl-(3.80%), 1b,5,5,6atetramethyl- octahydro-1-oxa-cyclopropa [a]inden-6- one (3.70%), 9,12-octadecadienoic acid (Z,Z)-(3.23%), 4,6-diisopropylidene-8,8-dimethyl-(2.86%), trans-Z-àbisabolene epoxide (2.86%), 2H-benzocyclohepten- 2-one, 1,4a,5,6,7,8,9,9a- octahydro-4a-methyl,trans-(2.78%), 2H-cyclopropa [g]benzofuran, 4,5,5a,6,6a,6b-hexahydro-4,4,6b-trimethyl-2-(1- methylethenyl)-(1.55%), 7-oxabicyclo [4.1.0]heptane, 1-methyl-4-(2- methyloxiranyl)-(1.50%), 6-octen-1- ol, 3,7-dimethyl-(1.42%), bicyclo [5.1.0]octan-2-one, 7-octene-1,2-diol (1.01%), 9-octadecenoic acid (Z)-, phenylmethyl ester (0.83%), 2,6,10-dodecatrien-1- ol, 3,7,11-trimethyl-, (Z,E)-(0.61%). The GC-MS spectrum of HAPB confirmed the presence of 22 compounds with the RT 7.07, 7.98, 8.12, 8.84, 9.58, 10.23, 10.69, 10.91, 11.09, 11.17, 12.30, 12.74, 13.20, 13.63, 13.92, 14.52, 15.10, 15.52, 16.19, 17.96, 22.91, 26.90, respectively (Figure 2).
The results revealed that D-mannose, alloaromadendrene oxide-(1), tetramethyl- octahydro-1,4,9,9-1H-3a,7- methanoazulene, limonene-1,2-epoxide, methyl ester of 2'-hexyl- [1,1'-bicyclopropyl]-2-octanoic acid, longipinene epoxide and aromadendrene oxide were found as a major components in HAPB. We found that the majority of compounds were terpenes and it was used for pesticide [27,28], antidiabetic, antidiarrheal effects [29], antiinflammatory [30,31], antioxidant, antileukemic, antitumor, anticancer, antiulcer, hepatoprotective [32-34].
Nowadays, the interest in the study of natural products is growing rapidly, especially as a part of drug discovery programs. It was concluded that the present mobile phase combination is suitable for eluting the phytoconstutuents from the extract. GC-MS analysis of HAPB concluded that the presence of various terpenes, terpenoids and fatty acids with varied degree. These various bioactive compounds present in the extract conform the application of P. barbatum for various diseases by traditional practitioners.
Thus, this type of GC-MS analysis is the preliminary step towards understanding the nature of active constituents present in the medicinal plants and this will be useful for further detailed study of plant. It could be concluded that, HAPB contains various bioactive components. So it is recommended as plant of pharmaceutical importance. However, further studies are needed to undertake its bioactivity and toxicity profile.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgement
We would like to acknowledge Mr. S. Kumaravel, Quality Manager, Food Testing Laboratory, Indian Institute of Crop Processing Technology (Ministry of Food Processing Industries, Government of India), Thanjavur, Tamil Nadu, India for his assistance to carry out GC-MS analysis of the sample.
Financial support
Nil.
References
- Wang KJ, Zhang YJ, Yang CR. Antioxidant phenolic compounds from rhizomes of Polygonum paleaceum. J Ethnopharmacol 2005;96:483-87.
- Islambekov SY, Karimdzhanov AK, Ismailov AI, Sadykov AS. Proanthocyanidins from Polygonum coriarium. Khim Prir Soedin 1969;5:325.
- Isobe T, Ito N, Noda Y. Minor flavonoids of Polygonum nodosum. Phytochemistry 1980;19:1877.
- Isobe T, Kanazawa K, Fujimura M, Noda Y. Flavonoids of Polygonum sieboldi and P. filiforme. Bull Chem Soc Jpn 1981;54:3239.
- Kang SS, Woo WS. Anthraquinones from the leaves of Polygonum sachalinense. Arch Pharm Res 1982;5:13-5.
- Nonaka G, Miwa N, Nishioka I. Stilbene glycoside gallates and proanthocyanidins from Polygonum multiflorum. Phytochemistry 1982;21:429-32.
- Ragazzl E, Froldl G, Fassina G. Resveratrol activity guinea pig isolated trachea from normal and albumin-sensitized animal. Pharmacol Res Commun 1988;20:79-82.
- Kim HJ, Woo ER, Park H. A novel lignin and flavonoids from Polygonum aviculare. J Nat Prod 1994;57:587-96.
- Brown LL, Larson SR, Sneden AT. Vanicosides C-F, new phenylpropanoid glycosides from Polygonum pensylvanicum. J Nat Prod 1998;61:762-66.
- Duwiejua M, Zeitlin IJ, Gray AI, Waterman PG. The anti-inflammatory compounds of Polygonum bistorta: Isolation and characterisation. Planta Med 1999;65:371-74.
- Sun X, Sneden AT. Neoflavonoids from Polygonum perfoliatum. Planta Med 1999;65:671-73.
- Datta BK, Datta SK, Rashid MA, Nash RJ, Sarker SD. A sesquiterpene acid and flavonoids from Polygonum viscosum. Phytochemistry 2000;54:201-05.
- Matsuda H, Shimoda H, Morikawa T, Yoshikawa M. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): Structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg Med Chem Lett 2001;11:1839-42.
- Murai Y, Kashimura S, Tamezawa S, Hashimoto T, Takaoka S, Asakawa Y, et al. Absolute configuration of (6S,9S)-roseoside from Polygonum hydropiper. Planta Med 2001;67:480-81.
- Peng ZF, Strack D, Baumert A, Subramaniam R, Goh NK, Chia TF, et al. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry 2003;62:219-28.
- Bhupinder Singh, Pandey VB, Joshi VK, Gambhir SS. Anti-inflammatory studies on polygonum glabrum. J Ethnopharmacol 1987;19:255-67.
- Hartwell JL. Plants used against cancer, A survey. Lloydia 1970;33:288-392.
- Lian LY. Studies on constituents of antihypertensive plant Polygonum perfoliatum. Kuo Li Chung – Kuo I Yao Yen Chiu So Yen Chiu Pao Kao 1983;7:103-29.
- Yim TK, Wu WK, Mak DHF, Ko KM. Myocardial protective effect of an anthraquinone containing extract of Polygonum multiflorum. Planta Med 1998;64:607-11.
- Kott V, Barbini L, Cruanes M, Munoz JD, Vivot E, Cruanes J, et al. Antiviral activity in Argentine medicinal plants. J Ethnopharmacol 1999;64:79-84.
- Kim HM. Antiallergy drugs from oriental medicines. Int J Orient Med 2000;1:1-7.
- Datta BK, Datta SK, Chowdhury MM, Khan TH, Kundu JK, Rashid MA, et al. Analgesic, antiinflammatory and CNS depressant activities of sesquiterpenes and a flavonoid glycoside from Polygonum viscosum. Pharmazie 2004;59:222-25.
- Wang KJ, Zhang YJ, Yang CR. Antioxidant phenolic compounds from rhizomes of Polygonum paleaceum. J Ethnopharmacol 2005;96:483-87.
- Lakshminarayanan B, Subburaju T, Pradeep Rajkumar LA, Manogaran E, Malairajan P, Satheesh Babu N, et al. Pharmacological screening of the anti-ulcerogenic effects of Polygonum barbatum (L) against gastric ulcers in rats. Inventi Impact-Ethinopharmacol 2011;4:1-4.
- Harborne JB. Phytochemical methods. 3rd ed. London: Chapman and Hall Ltd.; 1998.
- Wagner H, Baldt S. Plant drug analysis. Berlin: Springer; 1996.
- Mohan Das N, Sivakama Sundari S, Karuppusamy S, Mohan VR, Parthiban B. GC-MS analysis of leaf and stem bark of Cleidion nitidum (Muell. ARG.) THW. Ex Kurz. (Euphorbiaceae). Asian J Pharm Clin Res 2014;7:41-7.
- Herrera JM, Zunino MP, Dambolena JS, Pizzolitto RP, Ganan NA, Lucini EI, et al. Terpene ketones as natural insecticides against Sitophilus zeamais. Ind Crops Prod 2015;70:435-42.
- Khatun H, Nesa L, Islam R, Ripa FA, Al Mamum, Kadir S. Antidiabetic and antidiarrheal effects of the methanolic extract of Phyllanthus reticulatus leaves in mice. Asian Pac J Reprod 2014;3:121-27.
- Romero JC, Adriana MV, Herrera MP, Karina MM, Hortensia PD, Francisco JPF, et al. Synthesis anti-inflammatory activity and modelling studies of cycloartane-type terpenes derivatives isolated from Parthenium argentatum. Bioorg Med Chem 2014;22:6893-98.
- Ma JQ, Liu CM, Qin ZH, Jiang JH, Sun YZ. Ganoderma applanatum terpenes protect mouse liver against benzo(α)pyren-induced oxidative stress and inflammation. Environ Toxicol Pharmacol 2011;31:460-68.
- Fernandes ES, Rodrigues FA, Tófoli D, Imamura PM, Carvalho JE, Ruiz ALTG, et al. Isolation, structural identification and cytotoxic activity of hexanic extract, cyperenoic acid, and jatrophone terpenes from Jatropha ribifolia roots. Rev Bras Farmacogn 2013;23:441-46.
- Mendanha SA, Moura SS, Anjos JL, Valadares MC, Alonso A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol In Vitro 2013:27:323-29.
- Ma JQ, Liu CM, Qin ZH, Jiang JH, Sun YZ. Ganoderma applanatum terpenes protect mouse liver against benzo(α)pyren-induced oxidative stress and inflammation. Environ Toxicol Pharmacol 2011:31:460-68.