- *Corresponding Author:
- Swati Rawat
Y. B. Chavan College of Pharmacy, Rouza Bagh, Aurangabad - 431 001, India
E-mail: swati@k.st
| Date of Submission | 14 March 2005 |
| Date of Revision | 24 May 2007 |
| Date of Acceptance | 18 July 2007 |
| Indian J. Pharm. Sci., 2007, 69 (4): 529-534 |
Abstract
Complexing a drug may alter the rate and extent of drug absorption. The complex formation is very well applied in the administration of poorly water-soluble drugs. The drugs selected for the study are cyclooxygenase-2 inhibitors, are potent anti-inflammatory drugs with very low water solubility. The water solubility of these drugs was enhanced by complexing with β -cyclodextrin. In vitro absorption studies using isolated inverted bovine gut technique showed greater rate of transport of these drugs when complexed with β -cyclodextrin. The increase in the rate of transport is due to the formation of inclusion complexes with β -cyclodextrin that in turn increases the absorption. Studies also reveal that as the concentration of complexing agent increases the rate of absorption also increases proportionately. A statistical correlation was attempted between the mean percent drug dissolved at time 't' and quantity of drug absorbed at time 't/2'. When relation of in vitro drug dissolution and in vitro drug absorptions were studied, it was found that the r 2 -values for all formulations are within 0.947 to 0.997. This indicates a strong positive correlation between the in vitro drug dissolution and absorption of the drug through everted gut.
Keywords
Rofecoxib, celecoxib, meloxicam, cyclodextrin, inclusion complex
A number of different microorganism and plants produce certain enzymes called cyclodextrin glucosyltransferases, which degrade starch to cyclic products called cyclodextrins. These are cyclic oligosaccharide consisting of a lipophilic central cavity and a hydrophilic outer surface. Because of such characteristics cyclodextrins forms inclusion complex both in solution and in solid state, in which each guest molecule is surrounded by hydrophobic environment of the cyclodextrin cavity. This can lead to alteration of physical, chemical and biological properties of the guest molecules and can eventually have considerable pharmaceutical potential [1,2]. Out of the three parent cyclodextrins, β-cyclodextrin (β-CD) appears most useful pharmaceutical complexing agent because of its complexing ability, low cost and other properties [3]. Apart from the kneading [4], the solid drug can be complexed with β-CD by freeze-drying [5], spraydrying [6], co-evaporation [7] or by roll mixing [8]. Therefore, β-CD was selected to form inclusion complex with rofecoxib, celecoxib and meloxicam to enhance their solubility.
Rofecoxib [9,10], celecoxib [11-13] and meloxicam [14-16], are non-steroidal antiinflammatory analgesic drug that inhibit the activity of the enzyme cyclooxygenase, which is responsible for the formation of prostaglandin that cause inflammation, swelling, pain and fever. It is widely used for the treatment of inflammatory conditions associated with rheumatoid arthritis, respiratory tract infection, soft tissue and oral cavity infection. These are practically insoluble in water and as such their oral absorption is dissolution rate limited.
Materials and Methods
Lupin Laboratories (India) had kindly provided celecoxib and Ranbaxy Laboratories (India) had kindly provided rofecoxib and meloxicam. β-CD was obtained from Cavitron (USA) and sodium hydroxide was procured from Merck (India). All other reagents and chemicals used were of analytical grade.
All the solutions were prepared in distilled water. A pH meter (model L1-120, Elico Pvt. Ltd., India) and constant temperature water bath, 6-stage dissolution rate apparatus (Model-Electrolab Programmable tablet dissolution test Apparatus USP XXI/XXII, TDT-06P, India) with a paddle stirrer and a spectrophotometer (Shimadzu UV-2101PC, UV/Vis scanning spectrophotometer, Japan) were utilized.
Preparation of complexes
The kneading method has been adopted for the preparation of the complexes. In this method weighed quantity of β-CD was mixed with one-third volume of water to make a homogenous paste. In this homogenous paste the drug was added and continuously mixed for 30 min. The preparation was dried at 45o, pulverized and finally sieved through mesh #100. For physical mixture the drug and carrier were weighed in 1:1 ratio, mixed in a mortar and shifted through mesh #100.
Dissolution of drug: β-CD complexes
The in vitro dissolution studies of pure drug, physical mixture and inclusion complexes were carried out in 900 ml of 0.1 N HCl and phosphate buffer pH 7.4, using USP 6-stage dissolution rate apparatus with a paddle stirrer. Samples equivalent to 25 mg, 100 mg and 15 mg of rofecoxib, celecoxib and meloxicam, respectively were taken for the studies a speed of 50 rpm and a temperature of 37±0.5o were maintained in each test. Samples of dissolution media were withdrawn at 0, 10, 20 min for 1 h, filtered through Whatman filter paper no 41 and assayed for drug contents by measuring absorbance at 267 nm, 247 nm and 350 nm, respectively for rofecoxib, celecoxib and meloxicam [17].
Perfusion media and drug solutions
The absorption rate studies were performed in perfusion media prepared in accordance with Sardar et al [18]. The perfusion solution used for irrigating molar concentration of salts 1.45×10-1 M NaCl, 4.56×10-3 M KCl, 1.25×10-3 M CaCl2 and 5×10-3 M NaH2PO4. Drug and drug: βCD complexes were dissolved in prepared buffer of pH 7.2 in such a concentration that the drug concentration maintained in solutions to100 µg/ml at 37±1o. Drug and drug:β-CD complexes solutions were made isotonic by addition of 0.9% w/v sodium chloride to the solutions. The intrinsic solubilities are given in the Table 1.
| Drugs/complexes | Solubility in different medias (mg/ml) at 25±0.5° | |||||
|---|---|---|---|---|---|---|
| Water | Phosphate buffer |
Saline phosphate buffer |
Methanol | 0.1 N NCI | ||
| R | 0.0319 | 0.0184 | 0.0449 | 2.6587 | 0.0657 | |
| RPM | 0.0454 | 0.0524 | 0.0457 | 1.8734 | 0.0345 | |
| R | R1 | 0.0579 | 0.0930 | 0.0498 | 0.9843 | 0.0273 |
| X | R2 | 0.0649 | 0.0976 | 0.0502 | 0.9552 | 0.0199 |
| B | R3 | 0.0857 | 0.1278 | 0.0561 | 0.9387 | 0.0175 |
| R4 | 0.1766 | 0.1494 | 0.0697 | 0.9180 | 0.1880 | |
| C | 0.0151 | 0.0472 | 0.0050 | 1.0686 | 0.0129 | |
| CPM | 0.0165 | 0.0875 | 0.0062 | 1.0978 | 0.0125 | |
| C | C1 | 0.0188 | 0.1420 | 0.0081 | 1.2330 | 0.0121 |
| X | C2 | 0.0280 | 0.1320 | 0.0085 | 1.2386 | 0.0110 |
| B | C3 | 0.0312 | 1209 | 0.0090 | 1.0554 | 0.0117 |
| C4 | 0.0498 | 0.1068 | 0.0171 | 0.9599 | 0.0129 | |
| M | 0.0303 | 0.8105 | 0.8474 | 3.4645 | 0.0191 | |
| MPM | 0.0485 | 0.7822 | 0.8323 | 1.2543 | 0.0121 | |
| M | M1 | 0.0632 | 0.7416 | 0.8132 | 0.7161 | 0.0042 |
| X | M2 | 0.1094 | 0.7177 | 0.8036 | 0.7175 | 0.0033 |
| M | M3 | 0.3323 | 0.7126 | 0.7203 | 0.7137 | 0.0021 |
| M4 | 0.6527 | 0.7125 | 0.7005 | 0.7099 | 0.0018 | |
R stands for rofecoxib, R1 is rofecoxib:β-CD complex (1:0.25), R2 is rofecoxib: β-CD complex (1:0.5), R3 is rofecoxib:β-CD complex (1:1), and R4 is rofecoxib:β-CD complex (1:2)C= celecoxib, C1= celecoxib-βCD complexes (1:0.25), C stands for celecoxib, C1 is celecoxib:β-CD complex (1:0.25), C2 is rofecoxib:β-CD complex (1:0.5), C3 is celecoxib:β-CD complex (1:1), and C4 is celecoxib:β-CD complex (1:2). M stands for meloxicam, M1 is meloxicam:β-CD complex (1:0.25), M2 is meloxicam:β-CD complexes (1:0.5), M3 is meloxicam:β-CD complex (1:1) and M4 is meloxicam:β-CD complex (1:2).
Table 1: Determination of solubility of drugs and their complexes in different media at 25±0.5°
Absorption of drug and drug: β-CD complexes through intestinal membrane
The bovine intestine after isolation was immediately transferred to physiological salt solution (perfusion media) maintained at 37±1o with appropriate aeration (20 bubbles per min.). A portion of stomach was cut off from the isolated intestine. Each piece was washed thoroughly and its contents emptied completely by gentle flow of water. Its surface was made mucus-free, by gentle uni-directional movement between two fingers. After thorough washing, the gut was inverted, using a glass rod and thread. The solution with known concentration of drugs or their complexes in saline phosphate buffer pH 7.4 was prepared in a beaker. Using the biological tubing, cannulae and stand a gut piece was placed in a drug solution in such a way (like a U-band) that whole piece of everted intestine dipped in the drug solution. The solution was aerated (20 bubbles per min) using aeration pump and the temperature was maintained at 37±1o. With the help of a cannula fixed at the ends of the intestine, physiological salt solution was made to run continuously through it. The sample (2 ml) from mucosal side was withdrawn periodically and replaced with fresh drug solution. The drug, which was absorbed through the intestine, was assayed by periodical estimation of the drug in the withdrawal samples (mucosal side), using an UVspectrophotometric method. The decrease in drug concentration from its initial concentration gave the amount of drug absorbed.
Correlation of in vitro dissolution and in vitro absorption
To assess the correlation between the in vitro dissolution and in vitro absorption graphs were plotted between dissolution of drug in time t and absorption of the drug through the gut wall in t/2.
Results and Discussion
To perform the dissolution studies, the drug-complex equivalent to their doses were filled in the capsule and subjected for the dissolution tests. The dissolution studies (carried out in 900 ml of phosphate buffer pH 7.4, using USP 6-stage dissolution rate apparatus with a paddle stirrer) of rofecoxib:β-CD complexes exhibited that the dissolution rate increases with increasing concentration of β-CD in the complex (Table 2) and reaches to maximum dissolution rate (k=15.96×10-1 min-1) in phosphate buffer pH 7.4 when the complex prepared with 1:2 ratio of drug and β-CD, the saturation stage. The dissolution rate of the pure rofecoxib drug 3×10-1 min-1 and that of the physical mixture was 4.52×10-1 min-1. When complexed with β-CD in proportions of 1:0.25, 1:0.5 and 1:1 the dissolution rates were found to be 8.5×10-1 min-1, 10.3×10-1 min-1 and 13.8×10-1 min-1, respectively (Table 2).
| Ratio of drug and β-CD | Mean percentage of drug dissolved (±SD)* | DE30 (%) | Kx10-1 min-1 | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 30 | 45 | 60 | |||
| R | 2.88 | 3.74 | 5.36 | 8.77 | 10.25 | 12.11 | 3.75 | 6.23 |
| RPM | 3.28 | 5.33 | 9.42 | 11.69 | 13.09 | 15.85 | 4.52 | 8.55 |
| R1 | 8.77 | 9.13 | 10.36 | 12.56 | 17.29 | 19.15 | 8.48 | 7.98 |
| R2 | 10.44 | 11.69 | 13.42 | 15.31 | 19.75 | 22.82 | 10.25 | 8.93 |
| R3 | 12.43 | 15.36 | 18.76 | 24.66 | 27.61 | 32.55 | 13.75 | 10.21 |
| R4 | 16.07 | 18.76 | 21.66 | 28.57 | 33.04 | 35.17 | 15.96 | 10.58 |
| C | 1.23 | 2.10 | 3.30 | 4.27 | 7.22 | 12.31 | 2.15 | 4.98 |
| CPM | 2.75 | 3.92 | 5.11 | 7.23 | 11.14 | 17.73 | 2.75 | 6.07 |
| C1 | 4.22 | 5.46 | 6.32 | 10.85 | 14.46 | 21.62 | 3.16 | 6.57 |
| C2 | 6.35 | 7.54 | 9.64 | 14.75 | 18.32 | 24.31 | 6.45 | 7.99 |
| C3 | 8.45 | 9.67 | 12.64 | 18.37 | 22.83 | 29.41 | 9.89 | 8.88 |
| C4 | 9.33 | 10.80 | 15.58 | 21.24 | 25.80 | 32.04 | 14.25 | 9.69 |
| M | 11.25 | 24.11 | 35.25 | 54.15 | 74.35 | 90.46 | 19.17 | 13.05 |
| MPM | 26.11 | 43.15 | 54.25 | 71.18 | 83.88 | 93.25 | 30.01 | 14.08 |
| M1 | 48.19 | 61.57 | 68.37 | 80.13 | 88.41 | 95.25 | 55.23 | 14.47 |
| M2 | 58.99 | 73.25 | 82.11 | 89.22 | 93.28 | 98.34 | 65.45 | 15.07 |
| M3 | 67.25 | 81.88 | 89.25 | 95.25 | 99.73 | 100.25 | 76.66 | 15.30 |
| M4 | 76.25 | 86.53 | 93.25 | 99.26 | 101.14 | 102.14 | 85.25 | 15.36 |
R stands for rofecoxib, R1 is rofecoxib:β-CD complex (1:0.25), R2 is rofecoxib: β-CD complex (1:0.5), R3 is rofecoxib:β-CD complex (1:1), and R4 is rofecoxib:β-CD complex (1:2)C= celecoxib, C1= celecoxib-βCD complexes (1:0.25), C stands for celecoxib, C1 is celecoxib:β-CD complex (1:0.25), C2 is rofecoxib:β-CD complex(1:0.5), C3 is celecoxib:β-CD complex (1:1), and C4 is celecoxib:β-CD complex (1:2). M stands for meloxicam, M1 is meloxicam:β-CD complex (1:0.25), M2 is meloxicam:β-CD complexes (1:0.5), M3 is meloxicam:β-CD complex (1:1) and M4 is meloxicam:β-CD complex (1:2).
Table 2: Dissolution profile of inclusion complexes in phosphate buffer ph 7.4
Similarly, the dissolution rate of celecoxib and meloxicam was determined and found to increase as on increasing the concentration of β-CD in their complexes. The dissolution rate when studied in phosphate buffer pH 7.4 were found to be 2.2×10-1 min-1, 2.8×10-1 min-1, 3.2×10-1 min-1, 6.5×10-1 min-1, 9.9×10-1 min-1 and 14.3×10-1 min-1 with pure celecoxib drug, physical mixture and celecoxib:β-CD complexes with 1:0.25,1:0.5, 1:1 and 1:2 ratios, respectively (Table 2). When dissolution rate was performed in phosphate buffer pH 7.4 with meloxicam, and its complexes with β-CD showed dissolution rates 19.17×10-1 min-1 for pure drug, 30.01×10-1 min-1 for physical mixture, 55.2×10-1 min-1 for 1:0.25 complexes, 65.5×10-1 min-1 for 1:0.5 complex, 76.7×10-1 min-1 and for 1:1 complex and 85.3×10-1 min-1 for 1:2 complex (Table 2).
Drug dissolution from all the formulations followed first order kinetics as a straight line was obtained when log of drug remaining plotted against time. The corresponding dissolution efficiencies i.e., DE30% (dissolution efficiencies at 30 min) were also calculated as per Khan [19] are shown in the Table 2. Increased dissolution efficiency of drug was observed with increased β-CD concentration in the inclusion complexes when compared to the pure drug and the physical mixture.
When dissolution efficiency of pure rofecoxib compared with the dissolution efficiency of 1:2 complexes, it was found that the efficiency was increased by 4.3 fold. Similarly the dissolution efficiency of celecoxib and meloxicam were found to be 6.6 fold for celecoxib and 4.4 fold for meloxicam, when compared to the dissolution efficiency of pure drug at 30 min.
The increase in solubility or dissolution of these drugs can be explained as water molecules in the cavity of the cyclodextrin are in an energetically unfavored state because of the apolar nature of the cavity. Replacement of high-energy water molecules with a hydrophobic guest is therefore favored. The removal of high-energy molecule out of the cavity by displacement is the driving force behind the formation of an inclusion complex in aqueous solution. The association of guest molecule with the cyclodextrin is non-covalent in nature and involves many week intermolecular forces. The hydrophobic interactions between the hydrophobic part of the guest and the apolar cavity that involves dehydration of the hydrophobic guest molecule and its transfer into the cavity, increases the affinity for water, hence increases the solubility or dissolution.
When ANOVA was applied at 95% confidence level (α= 0.05) there was a significant difference in the release of drug with drug inclusion complex in comparison to the pure drug formulations.
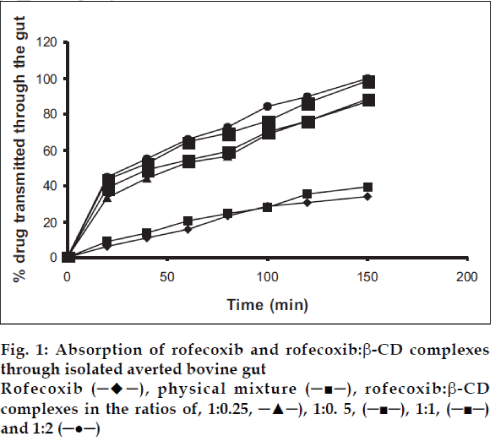
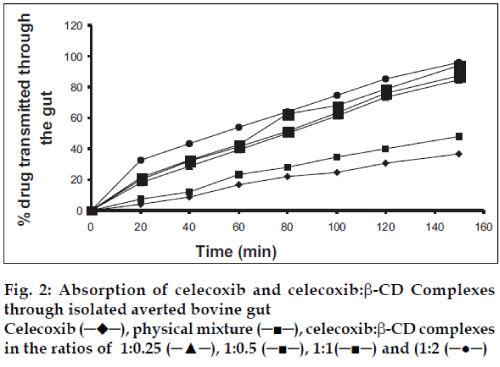
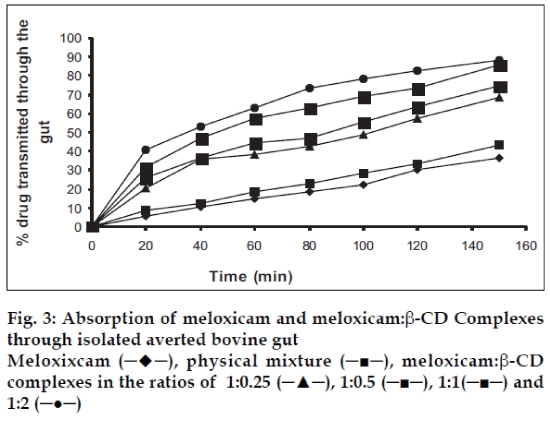
The absorption studies were performed using isolated everted intestinal sac model. Isolated inverted bovine gut model was set on the assumption that it follows first order absorption kinetics. Typical plots in figs. 1-3, show that amount of drugs (alone and as complexed) transported at various time intervals. The first order rate constant for the transport of drugs and in presence of β-CD are calculated. It can then be concluded that the increase in the solubility of drugs are due to formation of complexes with β-CD, which in turn increases the rate of transport or the rate of absorption. In the study it was observed that the absorption rates of complexes were higher than the pure drug and the physical mixtures.
It was observed that the absorption rates of 1:2 complexes (5.7×10-2 min-1) were higher than the pure drug (2.4×10-2 min-1) and the physical mixtures (2.6×10-2 min-1) of rofecoxib. Studies also reveal that as the concentration of complexing agent increases the rate of drug absorption also increases (5.0×10-2 min-1 for 1:0.25 complex, 5.2×10-2 min-1 for 1:0.5 complex and 5.5×10-2 min- 1for 1:1 complex). This is true for each case; the rates observed with celecoxib were 2.5×10-2 min-1for pure drug, 3.3×10-2 min-1 for the physical mixture, 5.6×10-2 min-1for 1:0.25 complex, 5.9×10-2 min-1 for 1:0.5 complex, 6.1×10-2 min-1 for 1:1 complex and 6.6×10-2 min-1 for 1:2 complex. The absorption rates for meloxicam were found to be 2.4×10-2 min-1for pure drug, 2.7×10-2 min-1for physical mixture, 4.0×10-2 min-1 for 1:0.25 complex, 4.9×10-2 min-1 for 1:0.5 complex, 5.1×10-2 min-1 for 1:1 complex and 5.2×10-2 min-1 for 1:2 complex, respectively.
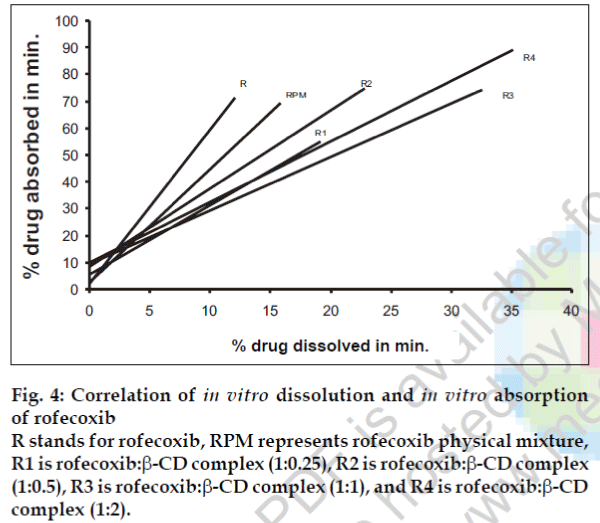
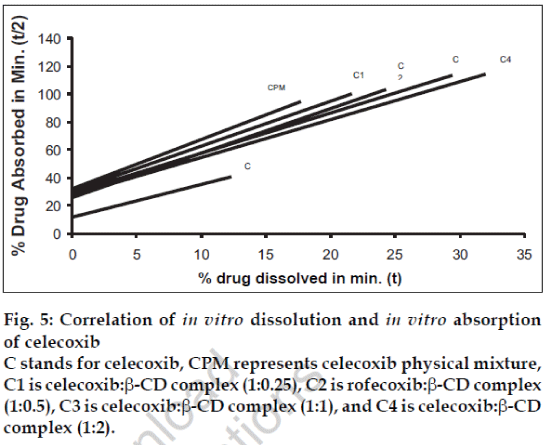
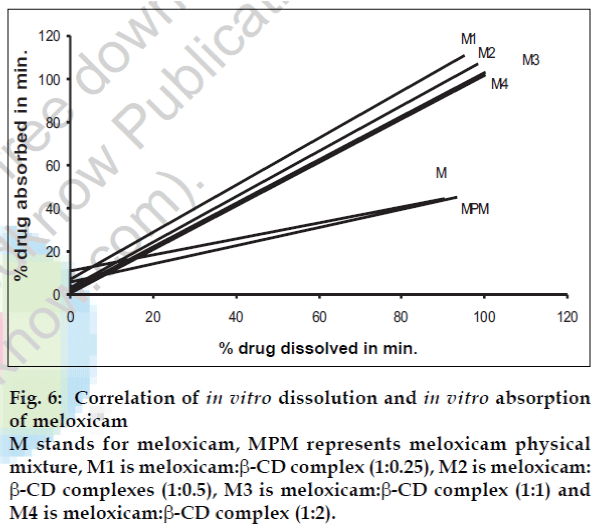
A statistical correlation was established between the mean percent drug dissolved at time ‘t’ and quantity of drug absorbed at time ‘t/2’. When relation of in vitro drug dissolution and in vitro drug absorptions were studied, it was found that the r2-values for all formulations are within 0.947 to 0.997. This indicates a strong positive correlation between the in vitro drug dissolution and absorption of the drug through isolated everted gut. The values are graphically represented in figs. 4 to 6.
Fig. 4: Correlation of in vitro dissolution and in vitro absorption of rofecoxib R stands for rofecoxib, RPM represents rofecoxib physical mixture, R1 is rofecoxib:β-CD complex (1:0.25), R2 is rofecoxib:β-CD complex (1:0.5), R3 is rofecoxib:β-CD complex (1:1), and R4 is rofecoxib:β-CD complex (1:2).
Fig. 5: Correlation of in vitro dissolution and in vitro absorption of celecoxib C stands for celecoxib, CPM represents celecoxib physical mixture, C1 is celecoxib:β-CD complex (1:0.25), C2 is rofecoxib:β-CD complex (1:0.5), C3 is celecoxib:β-CD complex (1:1), and C4 is celecoxib:β-CD complex (1:2).
Fig. 6: Correlation of in vitro dissolution and in vitro absorption of meloxicam M stands for meloxicam, MPM represents meloxicam physical mixture, M1 is meloxicam:β-CD complex (1:0.25), M2 is meloxicam: β-CD complexes (1:0.5), M3 is meloxicam:β-CD complex (1:1) and M4 is meloxicam:β-CD complex (1:2).
References
- Loftsson, T. and Masson, M., Int. J. Pharm., 2001, 225, 15.
- Uekama, K., Hirayama, F. and Irie, T., Chem. Rev., 1998, 98, 2045.
- Loftsson, T. and Brewster, ME, J. Pharm. Sci ., 2000, 85, 1017.
- Rajgopalan, N., Chen, S.C. and Chow, W.S ., Int. J. Pharm ., 1986, 29, 161.
- Yamamoto, M., Hirayama, F. and Uekama, K., Chem. Pharm. Bull ., 1992, 40, 747.
- Conte, U., Giunchendi, P., Magi, L. and La Manna, A., STD. Pharm. Sci ., 1993, 3, 42.
- Sanghvi, M., Choudhari, K.B., Matharu, R.S. and Viswanathan, L., Drug. Develop. Ind. Pharm. 1993, 19, 701
- Nozawa, Y. and Yarnamato, A., Pharm. Acta. Helv., 1989, 64, 24.
- Quiralte, J., Delgado, J., Saenz de San Pedro, B., Lopez-Pascual, E., Nieto, M.A., Ortega, N. Florido, J.F. and Conde, J., Ann. Allergy Asthma Immunol., 2004, 93, 360.
- Battisti, W.P., Katz, N.P., Weaver, A.L., Matsumoto, A.K., Kivitz, A.J., Polis, A.B. and Geba, G.P., J. Pain, 2004, 5, 511.
- Wang, K., Tarakji, K., Zhou, Z., Zhang, M., Forudi, F., Zhou, X., Koki, A.T., Smith, M.E., Keller, B.T., Topol, E.J., Lincoff, A.M. and Penn, M.S., J. Cardiovasc. Pharmacol., 2005, 45, 61.
- Kiefer, W. and Dannhardt, G., Curr. Med. Chem ., 2004, 11, 3147.
- Thakkar, H., Sharma, R.K., Mishra, A.K., Chuttani, K. and Murthy, R.S., J. Drug Target. 2004, 12, 549.
- Naidu, N.B., Choudhari, K.P., Murthy, K.V., Satyanarayana, V., Hayman, A.R. and Becket, G., J . Pharm. Biomed Anal., 2004, 35, 75.
- Santos, M., Kunkar, V., Garcia-Iturralde, P. and Tendillo, F.J., Anesth. Analg., 2004, 98, 359.
- Seedher, N. and Bhatia, S., AAPS Pharm. Sci. Tech., 2003, 4, E33.
- Carstensen, J.T., Lai, T.Y. and Prasad, V.K., J. Pharm. Sci., 1978, 7, 1303.
- Sardar, A.A., Murugan, J.S., Shameem, S.M., Shanmugapriya, P., Sriramkumar, S., Vijayaraghavan, C. and Divakar, M.C., Indian Drugs , 1999, 36, 225.
- Khan K.A., J. Pharm. Pharmacol ., 1972, 27, 48.