- *Corresponding Author:
- C. Wang
Department of Dermatology, Taipei Tzuchi Hospital, The Buddhist Tzuchi Medical Foundation, New Taipei City 231, Taiwan
E-mail: dermawang@gmail.com
| Date of Submission | 26 October 2016 |
| Date of Revision | 19 April 2017 |
| Date of Acceptance | 01 December 2017 |
| Indian J Pharm Sci 2018;80(1):126-134 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Tournefortia sarmentosa is a Chinese herbal medicine used as an antioxidant, detoxicant, and anti-inflammatory agent. The present study evaluated the effect of T. sarmentosa on adenosine diphosphate-induced platelet aggregation. Our results showed that aqueous stem extract of T. sarmentosa inhibited adenosine diphosphate-induced platelet aggregation. In addition, we found components in T. sarmentosa, including caffeic acid, rosmarinic acid, salvianolic acid, play important roles in mediating adenosine diphosphate-induced platelet aggregation suppression. The stem extract of T. sarmentosa inhibited the adenosine diphosphate receptor P2Y12-mediated cyclic AMP production. Caffeic acid inhibited P2Y1-induced calcium influx. Furthermore, treatment of platelets with T. sarmentosa, or the components in stem extract of T. sarmentosa, suppressed adenosine diphosphate-induced release of thromboxane A2 and arachidonic acid and surface PAC-1 expression. These data demonstrate the aqueous stem extract of T. sarmentosa significantly suppressed platelet aggregation through P2Y1 and P2Y12 receptor signal pathways. The antiaggregation properties found in the stem extract of T. sarmentosa might help to prevent cardiovascular disease.
Keywords
Tournefortia sarmentosa, platelet, caffeic acid, rosmarinic acid, salvianolic acid
Tournefortia sarmentosa Lam. (Boraginaceae) is a Chinese herbal medicine with antioxidant activities, detoxicating qualities and antiinflammatory uses [1-3]. Numerous pharmacological observations have shown therapeutic effects. Lin et al. revealed that isolated components from the stems of T. sarmentosa decreased Cu2+-induced low-density-lipoprotein oxidation [1]. Teng et al. investigated the hepatotoxicity effects of T. sarmentosa stem extract and found that it reduced elevated levels of liver function markers, including serum glutamate oxaloacetate transaminase (SGOT), glutamate pyruvate transaminase, and alkaline phosphatase (ALP). It also reduced levels of inflammatory markers, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in acetaminophen-intoxicated rats [2]. However, some results measured the inflammatory cytokine expression in cells treated with T. sarmentosa stem extract are contradictory. For example, extracts of T. sarmentosa enhanced the release of cytokines, including IL-6, IL-8, and TNF-α [4] and increased phagocytosis by macrophages and neutrophils [4-5].
Various pharmacologically active compounds from aqueous stem extract of T. sarmentosa have been identified [3] and these components play roles in immune or detoxification regulation. For example, salvianolic acid A, isosalvianolic acid C, and rosmarinic acid exhibited roles in reducing reactive oxygen species activity [1]. Caffeic acid increased the phagocytic ability of macrophages and neutrophils by increasing phosphorylated p38 MAPK or AKT, respectively [4-5]. Salvianolic acid inhibited phagocytic ability of macrophages by suppressing lipopolysaccharideinduced ERK1/2 phosphorylation [4]. These results suggest that each of the components in aqueous stem extract of T. sarmentosa possesses a modulating role in regulating macrophage/neutrophil cells.
The main physiological function of platelets is to stop bleeding by clumping and clotting blood vessel injuries. In addition to coagulation factors (thrombin), collagen and hormones (epinephrine), adenosine diphosphate (ADP) have been well identified as a contributor to the propagation of platelet activation at sites of vascular injury. The receptors that mediate signalling of ADPinduced platelet aggregation have been thoroughly described using whole blood or platelet-rich plasma (PRP) [6-10]. Previous studies demonstrated that two main G protein-coupled receptors; P2Y1 and P2Y12, are involved in the ADP receptor signalling of platelet activation [11-14]. Gq-coupled ADP receptor P2Y1 leads to the activation of phospholipase Cβ, and subsequently induces intracellular calcium mobilization and activates protein kinase C. P2Y12 receptor activated by ADP and couples with Gi to inhibit adenylyl cyclase and activate PI3-kinase [12,15,16]. P2Y12 receptor is essential for the release of arachidonic acid from membranebound phospholipids, and subsequent thromboxane A2 (TXA2) generation catalysed by cyclooxygenase in platelets [17]. TXA2 functions as an amplified mediator in platelet activation [18,19]. While normal blood clots form a protective seal over an injury, abnormalities of the blood clotting are significant causes of illness and death. The most common form of heart attack occurs when a blood clot blocks the blood flow through the heart muscle. Also, the leading cause of stroke occurs when a blood clot blocks an artery supplying blood to the brain. Antiplatelet drugs are most effective for arterial clots and are useful in preventing cardiovascular diseases.
T. sarmentosa has been reported to function as an antioxidant and enhance inflammatory responses. However, there have been no reports on the action of T. sarmentosa against platelet activity. In this study, we investigated the regulatory effects of aqueous stem extract of T. sarmentosa, and the major components in stem extract of T. sarmentosa, on ADP-induced platelet aggregation.
Materials and Methods
The stems of T. sarmentosa Lam. were collected from Jhongpu Township, Chiayi County, Taiwan and the extraction of T. sarmentosa have been described previously [4,5]. Briefly, the dried stems of T. sarmentosa were grinded into powder and 100 g of T. sarmentosa stem powder were macerated with hot water (95°) for 2 h. The aqueous extract was filtered under vacuum at 70°. The extracted solution was centrifuged at 4° and 9000 rpm for 15 min, and then lyophilized to obtain dry powder and stored at −20° before use. The ADP, forskolin, fibrinogen, caffeic acid, rosmarinic acid, salvianolic A, and salvianolic B were obtained from Sigma-Aldrich (St. Louis, USA). The adenosine 2',5'-diphosphate (A2P5P) and clopidogrel were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of human platelets.
Human blood was obtained by venipuncture from healthy adults and collected in a vacutainer containing sodium citrate. Blood was centrifuged for 20 min at 180×g at room temperature to get PRP. Samples of PRP (2×108 platelets/ml) were preincubated with T. sarmentosa stem extract, caffeic acid (100 μM), rosmarinic acid (100 μM), salvianolic A (100 μM), or salvianolic B (100 μM) at 37° for 30 min. PRP were then treated with A2P5P or clopidogrel for 3 min and 10 μM of ADP was added. Alternatively, washed platelets and platelet poor plasma (PPP) were obtained from PRP by centrifugation for 15 min at 1500×g. The supernatant was PPP and the pellet (washed platelets) was resuspended to a density of 2×108 platelets/ml in a modified calcium-free Tyrode buffer (138 mM NaCl; 2.7 mM KCl; 1 mM MgCl2; 3 mM NaH2PO4; 10 mM HEPES; 5 mM glucose; 0.2 % BSA; and 20 μg/ml apyrase, pH 7.4). All subjects signed a consent form for participation in the study and our study was reviewed and approved by Taipei Tzuchi Hospital, the Buddhist Tzuchi Medical Foundation Institutional Review Board.
Measurement of platelet aggregation
Platelet aggregation was measured in PRP by the turbidimetric method in duplicate, using a light transmittance aggregometer (PAP-8E, Platelet Aggregation Profiler, Bio/Data Corporation, Horsham, PA). Briefly, ADP-induced platelet aggregation was monitored using an aggregometer at 37° under continuous stirring. Baseline optical density was set using the PPP sample. Aggregation was induced by addition of 10 μM ADP and monitored for 6 min by optical aggregometry.
Measurement of intracellular calcium in platelets
PRP was pre-treated with T. sarmentosa stem extract or each compound (100 μM) for 30 min. About 3 μM Fura-2AM (Molecular Probes, Invitrogen, Carlsbad, CA) was added to the PRP and incubated at 37° for 45 min as mentioned in an aggregometer at 37° with stirring at 900 rpm. Platelets were centrifuged and resuspended in modified calcium-free Tyrode buffer. ADP (10 μM)-induced calcium responses were subsequently measured at 37° in a multi-functional microplate reader (Infinite F200, Tecon, Durham, NC) with fluorescence excitation set at 340 nm and 380 nm, emission was set to 510 nm.
Measurement of cyclic AMP (cAMP) levels and TXA2 in platelets
PRP was treated with T. sarmentosa stem extract or each compound (100 μM) for 30 min. Washed platelets were prepared in modified calcium-free Tyrode buffer and then incubated with 10 μM forskolin and 10 μM ADP at 37° for 5 min as mentioned in an aggregometer at 37° with stirring at 900 rpm. After washing twice with PBS, cells were lysed with 0.1 N HCl, scraped, and collected by centrifugation. Levels of cAMP in the supernatants were determined using a cAMP enzyme immunoassay (EIA) kit according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI).
PRP was treated with T. sarmentosa stem extract or each compound (100 μM) for 30 min. Washed platelets were prepared and then stimulated with 3 μM fibrinogen and 10 μM ADP at 37° for 3 min as mentioned, in an aggregometer at 37° with stirring at 900 rpm. The reaction was stopped by quickly freezing the sample in a dry ice-ethanol bath. After thawing at room temperature, the samples were centrifuged at 3000×g for 10 min at 4°. The supernatants were collected and the content of thromboxane B2, the stable metabolite of TXA2, was measured using an EIA according to the manufacturer’s instructions (Cayman Chemical).
Measurement of arachidonic acid release in platelets
PRP was treated with T. sarmentosa stem extract or each compound (100 μM) for 30 min. Washed platelets were prepared and then activated with 10 μM ADP and 3 μM fibrinogen at 37° for 3 min as mentioned in an aggregometer at 37° with stirring at 900 rpm. Levels of arachidonic acid in the supernatants were determined using a human arachidonic acid ELISA kit (Cusabio Biotech, Wuhan, Hubei, China).
Measurement of platelet activation markers expression
Whole blood was diluted 1:2 with PBS. The diluted whole blood (20 μl) was treated with T. sarmentosa stem extract or each compound (100 μM) for 30 min. Diluted whole blood was stained with phycoerythrin or fluorescein isothiocyanate-conjugated antibodies targeting CD61 (BD Pharmingen, San Jose, CA) and PAC-1 (BD Pharmingen) along with 20 μM ADP for 20 min at room temperature. The labelled whole blood were immediately fixed with 400 μl of 1 % (W/V) paraformaldehyde at room temperature for 30 min and subjected to flow cytometry analysis (FACScan, Becton Dickinson, Flanklin Lakes, NJ).
Statistical analysis
All values are expressed as mean±SD of at least triplicate samples. Statistical analyses were assessed using one-way ANOVA with Dunnett's post hoc test for comparison of more than two groups. A p-value <0.05 was considered statistically significant.
Results and Discussion
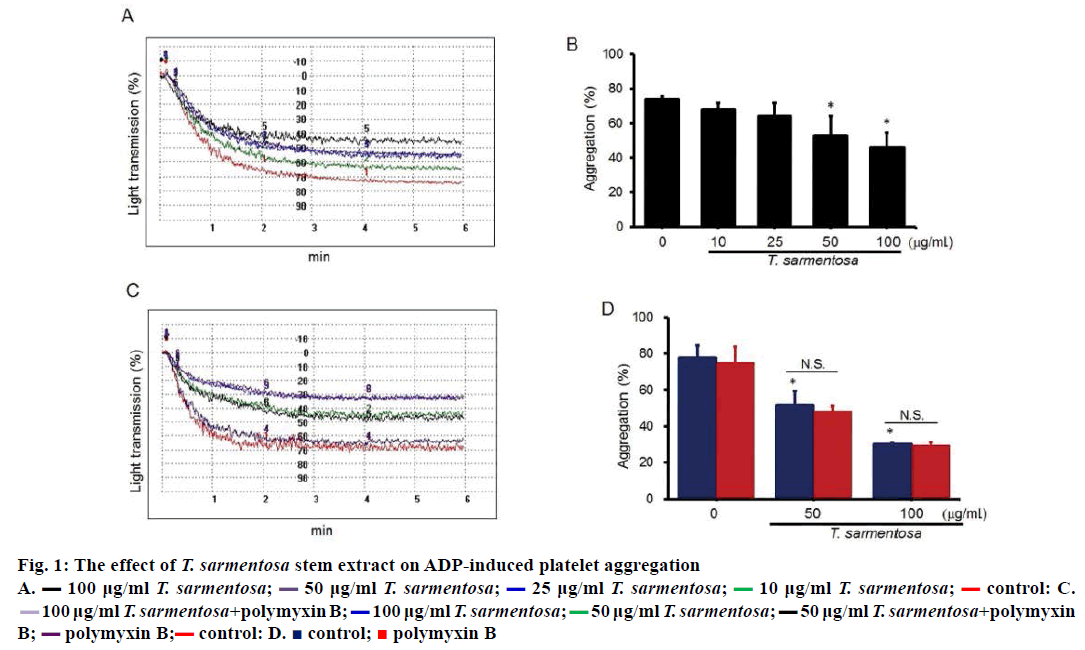
To examine the effect of T. sarmentosa on human platelet aggregation, human PRP were treated with various doses of T. sarmentosa stem extract for 30 min. After T. sarmentosa stem extract treatment, platelet aggregation was examined using an aggregometer. Results shown ADP-induced aggregation was suppressed in a dose-dependent manner, with maximal inhibition (37.8 %) in cells treated with 100 μg/ml T. sarmentosa stem extract (Figure 1A and B). To sort out the possibility of contamination of polysaccharides in the extract to exhibit antiaggregative activity, PRP were pre-treated with polymyxin B. We found the suppressive platelet aggregation by T. sarmentosa stem extract was not affected by treating platelet with polymyxin B (Figure 1C and D). The results indicate that T. sarmentosa stem extract exhibited antiaggregative ability.
Figure 1: The effect of T. sarmentosa stem extract on ADP-induced platelet aggregation
A.  100 μg/ml T. sarmentosa;
100 μg/ml T. sarmentosa;  50 μg/ml T. sarmentosa;
50 μg/ml T. sarmentosa;  25 μg/ml T. sarmentosa;
25 μg/ml T. sarmentosa;  10 μg/ml T. sarmentosa;
10 μg/ml T. sarmentosa;  control: C.
control: C.  100 μg/ml T. sarmentosa+polymyxin B;
100 μg/ml T. sarmentosa+polymyxin B;  100 μg/ml T. sarmentosa;
100 μg/ml T. sarmentosa;  50 μg/ml T. sarmentosa;
50 μg/ml T. sarmentosa;  50 μg/ml T. sarmentosa+polymyxin
B;
50 μg/ml T. sarmentosa+polymyxin
B;  polymyxin B;
polymyxin B; control: D.
control: D.  control;
control;  polymyxin B
polymyxin B
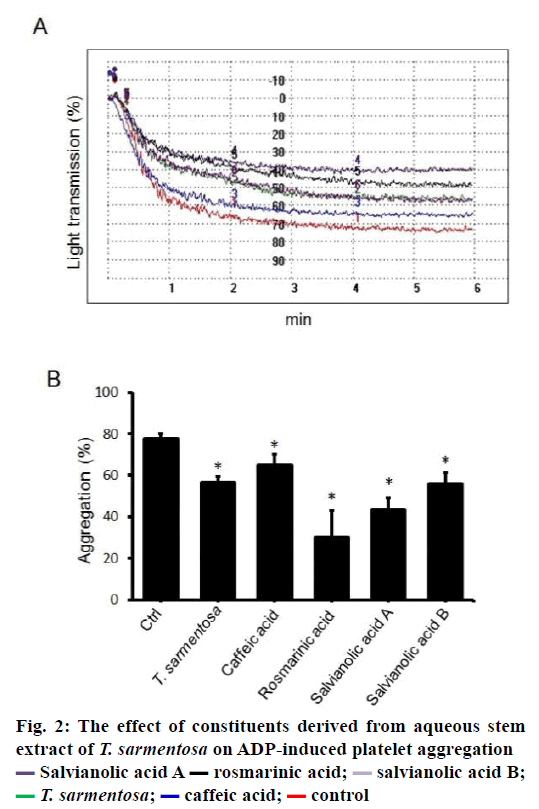
We also determined the effects of commercially available compounds, found in aqueous stem extract of T. sarmentosa on the aggregative capability of human platelets. Results show when human PRP were treated with T. sarmentosa stem extract, and the components studied of T. sarmentosa stem extract, ADP-induced platelet aggregation was suppressed. T. sarmentosa stem extract, rosmarinic acid, salvianolic acid A and salvianolic B significantly suppressed aggregation by 27.04 % to 61.37 %, and the caffeic acid had the weakest effect (the aggregative ability was decreased by 16.3 %) on ADP-induced platelet aggregation suppression among the major components in stem extract of T. sarmentosa tested (Figure 2A and B).
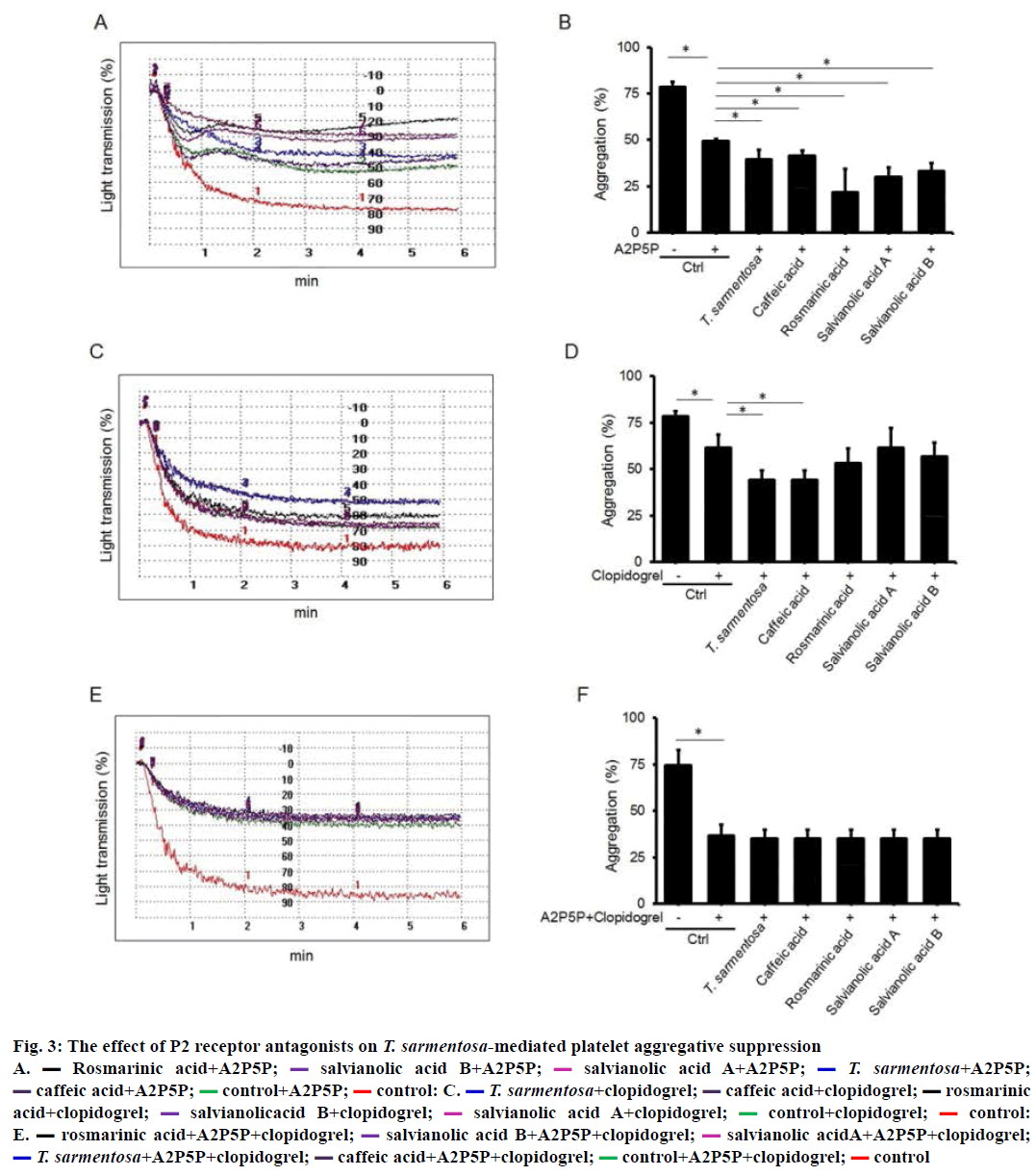
Two G-protein-coupled receptors, P2Y1 and P2Y12, play a central role in ADP-mediated platelet aggregation. To explore the potential signalling involved in T. sarmentosa-suppressed platelet aggregation, we treated PRP with P2 receptor antagonists. As shown in Figure 3A and B, ADP-induced platelet aggregation was blocked by 37 % by the P2Y1 receptor antagonist A2P5P (1 mM). Pretreatment with 50 μg/ml T. sarmentosa stem extract, and the components studied of T. sarmentosa stem extract (100 μM), significantly reduced platelet aggregation by 15.5 % to 56 % in platelets incubated with A2P5P. ADP-induced platelet aggregation was blocked by 21.7 % in PRP pretreated with P2Y12 receptor antagonist clopidogrel (30 μM). T. sarmentosa stem extract and caffeic acid further blocked platelet aggregation by 34 % in platelets incubated with clopidogrel (Figure 3C and D). The clopidogrel-mediated ADP-induced platelet aggregative suppression was not affected by PRP pretreated with rosmarinic acid, salvianolic A, or salvianolic B (Figure 3C and D). ADP-induced platelet aggregation was decreased by 50.9 % in platelets pretreated with A2P5P and clopidogrel. We found that neither T. sarmentosa stem extract nor the components studied of T. sarmentosa stem extract affected platelet aggregative ability when PRP pretreated with A2P5P and clopidogrel (Figure 3E and F). There results suggest that T. sarmentosa stem extract and caffeic acid inhibited platelet aggregation via P2Y1 and P2Y12 receptors. And, rosmarinic acid, salvianolic A, and salvianolic B blocked platelet aggregation through P2Y12 receptor.
Figure 3: The effect of P2 receptor antagonists on T. sarmentosa-mediated platelet aggregative suppression
A.  Rosmarinic acid+A2P5P;
Rosmarinic acid+A2P5P; salvianolic acid B+A2P5P;
salvianolic acid B+A2P5P;  salvianolic acid A+A2P5P;
salvianolic acid A+A2P5P;  T. sarmentosa+A2P5P;
T. sarmentosa+A2P5P;  caffeic acid+A2P5P;
caffeic acid+A2P5P;  control+A2P5P;
control+A2P5P;  control: C.
control: C.  T. sarmentosa+clopidogrel;
T. sarmentosa+clopidogrel;  caffeic acid+clopidogrel;
caffeic acid+clopidogrel;  rosmarinic
acid+clopidogrel;
rosmarinic
acid+clopidogrel;  salvianolicacid B+clopidogrel;
salvianolicacid B+clopidogrel;  salvianolic acid A+clopidogrel;
salvianolic acid A+clopidogrel;  control+clopidogrel;
control+clopidogrel;  control:
E.
control:
E.  rosmarinic acid+A2P5P+clopidogrel;
rosmarinic acid+A2P5P+clopidogrel;  salvianolic acid B+A2P5P+clopidogrel;
salvianolic acid B+A2P5P+clopidogrel;  salvianolic acidA+A2P5P+clopidogrel;
salvianolic acidA+A2P5P+clopidogrel;  T. sarmentosa+A2P5P+clopidogrel;
T. sarmentosa+A2P5P+clopidogrel;  caffeic acid+A2P5P+clopidogrel;
caffeic acid+A2P5P+clopidogrel;  control+A2P5P+clopidogrel;
control+A2P5P+clopidogrel;  control
control
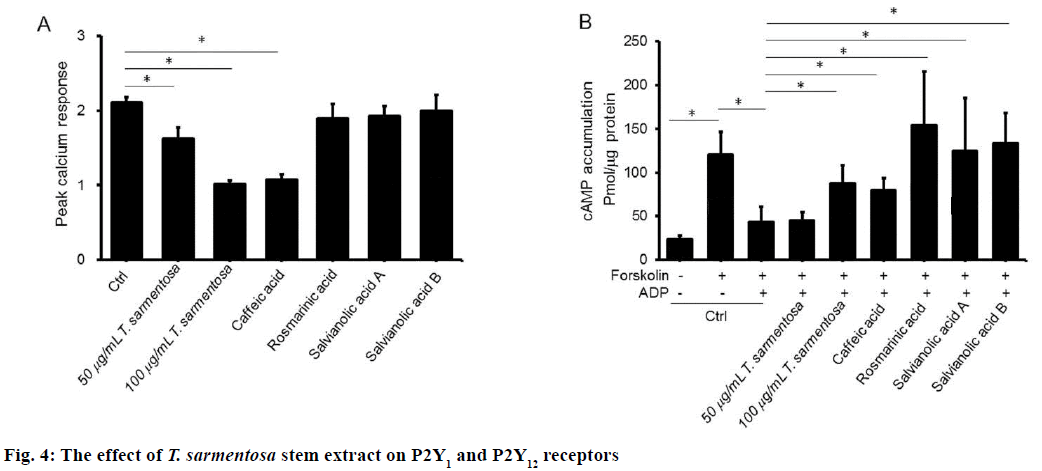
To further understand the mechanism involved in T. sarmentosa-mediated platelet aggregation suppression, we examined the effect of T. sarmentosa stem extract on ADP-induced calcium influx and cAMP suppression in PRP. Our data show reduced calcium responsiveness ranging from 23.1 % to 52 % in PRP pretreated with T. sarmentosa stem extract and caffeic acid (Figure 4A). Rosmarinic acid, salvianolic A, and salvianolic B had no effect on the ADP-induced calcium influx. In addition, a reduction of cAMP production was observed in forskolin-stimulated treated with ADP.
Preincubation with T. sarmentosa reverted the cAMP production in a dose-dependent manner in forskolininduced PRP treated with ADP (Figure 4B). Similarly, an increased in cAMP was observed in forskolin-and ADP-treated PRP pre-incubated with the components studied of T. sarmentosa stem extract.
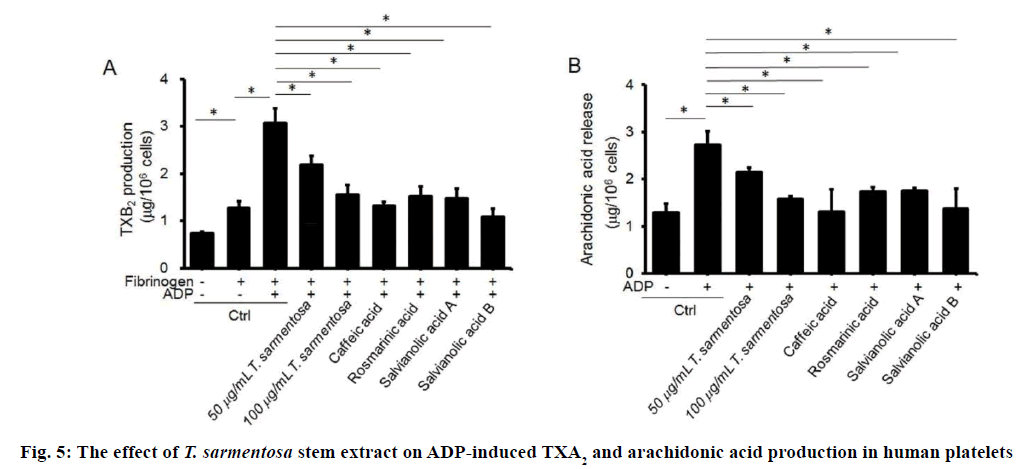
The TXA2 released during platelet aggregation stimulates activation of new platelets. To explore the effects of T. sarmentosa on ADP-enhanced platelet aggregation, we measured the expression of TXB2, the stable metabolite of TXA2. To induce TXB2 production, PRP were stimulated with fibrinogen (30 μM) and ADP. As expected, T. sarmentosa stem extract inhibited TXB2 production in a dose-dependent manner. Similar inhibitory activity was observed in PRP pre-treated with the components studied of T. sarmentosa stem extract (Figure 5A). TXA2 is generated by activated platelets, which convert arachidonic acids through the cyclooxygenase pathway. We determined the effect of T. sarmentosa stem extract on ADPinduced arachidonic acid production. As expected, decreased arachidonic acid production was observed in ADP-induced PRP treated with T. sarmentosa stem extract or the components studied of T. sarmentosa stem extract (Figure 5B).
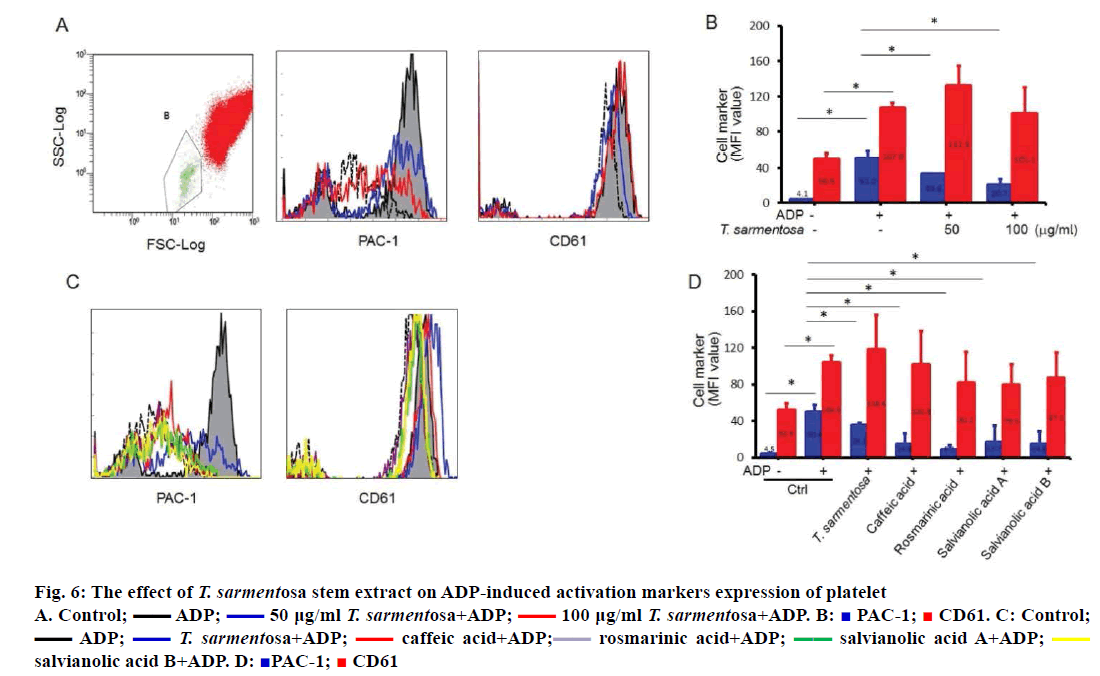
Glycoproteins found on platelets play crucial roles in platelet-mediated aggregation and interactions with the extracellular matrix. The glycoprotein complex IIb/IIIa changes its conformation leading to binding of adhesive proteins that contain RGD motif during platelet activation. Whole blood flow cytometry can analyse the exposed glycoproteins on the surface of platelets and measure platelet activation in vivo. Therefore, in order to elucidate the antiaggregative ability of T. sarmentosa stem extract on platelets in vivo, we detected the expression of surface CD61 (glycoprotein IIIa) and PAC-1 (glycoprotein IIb/ IIIa). As shown in Figure 6A and 6B, the surface CD61 expression of platelets was slightly enhanced by 1.1-fold; PAC-1 was significantly increased by 11.4- fold upon ADP addition. We observed dose-dependent decrease of surface PAC-1 expression in T. sarmentosa stem extract-treated whole blood. Results show it did not affect the surface CD61 expression of platelets (Figure 6A and B). We examined the effect of the components studied of T. sarmentosa stem extract on the surface CD61 and PAC-1 expression of platelets. Treatment of whole blood with the components studied of T. sarmentosa stem extract significantly decreased surface PAC-1 expression by 65 % to 82 %. Similarly, the components studied of T. sarmentosa stem extract had no effect on the surface CD61 expression of platelets (Figure 6C and D).
Figure 6: The effect of T. sarmentosa stem extract on ADP-induced activation markers expression of platelet
A. Control;  ADP;
ADP;  50 μg/ml T. sarmentosa+ADP;
50 μg/ml T. sarmentosa+ADP;  100 μg/ml T. sarmentosa+ADP. B:
100 μg/ml T. sarmentosa+ADP. B:  PAC-1;
PAC-1;  CD61. C: Control;
CD61. C: Control;  ADP;
ADP;  T. sarmentosa+ADP;
T. sarmentosa+ADP;  caffeic acid+ADP;
caffeic acid+ADP; rosmarinic acid+ADP;
rosmarinic acid+ADP;  salvianolic acid A+ADP;
salvianolic acid A+ADP;  salvianolic acid B+ADP. D:
salvianolic acid B+ADP. D:  PAC-1;
PAC-1;  CD61
CD61
The results here demonstrate that aqueous stem extracts of T. sarmentosa significantly decreased ADP-induced platelet aggregation. Also, aqueous stem extract of T. sarmentosa alleviated ADP-mediated calcium influx and production of cAMP, TXA2, and arachidonic acid in platelets and the activation surface marker PAC-1 expression of platelets. These findings suggest that aqueous stem extract of T. sarmentosa affects platelet activity.
The constituents of the aqueous extract of T. sarmentosa stems were further investigated in T. sarmentosamediated suppression platelet aggregation. Caffeic acid decreased Ca2+ mobilization and elevated cAMP levels in ADP-stimulated platelets [20]. Rosmarinic acid has been reported to possess antithrombotic and antiplatelet effects [21,22]. Both salvianolic A and B have been shown to inhibit platelet activation [23,24]. Taken together, these findings demonstrate T. sarmentosa stem extract suppressed platelet aggregation.
Although the individual components showed similar antiaggregative abilities, the molecular mechanisms for ADP-mediated platelet activity were somewhat different for each component studied of T. sarmentosa stem extract. The cAMP production is affected by all components studied of T. sarmentosa stem extract. Calcium influx was inhibited by caffeic acid and aqueous stem extract of T. sarmentosa. However, rosmarinic acid, salvianolic A, and salvianolic B did not. All components studied of T. sarmentosa stem extract significantly inhibited the ADP-induced production of TXA2, arachidonic acid and the surface marker PAC-1 on platelets.
Caffeic acid plays an important role in T. sarmentosamediated regulation of phagocytic uptake of neutrophils and macrophages through Akt/MEK pathway [4-5]. Fewer reactive oxygen species were observed in T. sarmentosa treated macrophages, particularly with salvianolic acid A and salvianolic B [4]. The present study shows that all components studied in T. sarmentosa suppressed platelet aggregation, indicating each component studied mediated the effects of T. sarmentosa on different cells in various ways.
Antiplatelet drugs are widely used in primary and secondary prevention of thrombotic cerebrovascular and cardiovascular disease. Antiplatelet drugs are classified as reversible or irreversible, according to the inhibition process involved in platelet activation. Aspirin is an irreversible inhibitor of enzyme cyclooxygenase, resulting in reduced platelet production of TXA2 [25]. Dipyridamole is a phosphodiesterase inhibitor that increases the cyclic AMP by preventing conversion to AMP in platelets [26]. Clopidogrel affects the ADPdependent activation of IIb/IIIa complex via ADP receptor P2Y12 [27]. Glycoprotein IIb/IIIa receptor antagonists, including abciximab and eptifibatide, block a receptor on platelets for fibrinogen and von Willebrand factor [28]. A daily low dose of aspirin is considered safe at reducing the risk of heart attacks and strokes [29]. Given that T. sarmentosa enhances the immune response [4,5], prevents chemical-induced hepatotoxicity [2], and suppresses platelet aggregation, it is considered a traditional Chinese herb promoting health.
Acknowledgements
The authors thank the Core Laboratory of the Buddhist Tzuchi General Hospital for support.
Financial support and sponsorship
This work was supported by Grant (TCRDTPE- 106-01) of the Taipei Tzuchi Hospital through the Buddhist Tzuchi Medical Foundation, Taipei, Taiwan.
Conflicts of interest
There are no conflicts of interest.
References
- Lin YL, Chang YY, Kuo YH, Shiao MS. Anti-lipid-peroxidative principles from Tournefortia sarmentosa. J Nat Prod 2002;65:745-7.
- Teng CY, Lai YL, Huang HI, Hsu WH, Yang CC, Kuo WH. Tournefortia sarmentosa extract attenuates acetaminophen-induced hepatotoxicity. Pharm Biol 2012;50:291-396.
- Lin YL, Tsai YL, Kuo YH, Liu YH, Shiao MS. Phenolic compounds from Tournefortia sarmentosa. J Nat Prod 1999;62:1500-03.
- Chen ML, Wu S, Tsai TC, Wang LK, Chou WH, Tsai F. Effect of aqueous extract of Tournefortia sarmentosa on the regulation of macrophage immune response. Int Immunopharmacol 2013;17:1002-8.
- Chen ML, Wu S, Tsai TC, Wang LK, Chou WH, Tsai FM. The caffeic acid in aqueous extract of Tournefortia sarmentosa enhances neutrophil phagocytosis of Escherichia coli. Immunopharmacol Immunotoxicol 2014;36:390-6.
- Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag 2015;11:133-48.
- Harrison P, Mackie I, Mumford A, Briggs C, Liesner R, Winter M, et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol 2011;155:30-44.
- Choi JL, Li S, Han JY. Platelet function tests: a review of progresses in clinical application. Biomed Res Int 2014;2014:456569.
- Sudo T, Ito H, Kimura Y. Characterization of platelet aggregation in whole blood of laboratory animals by a screen filtration pressure method. Platelets 2003;14:239-46.
- Soloviev MV, Okazaki Y, Harasaki H. Whole blood platelet aggregation in humans and animals: a comparative study. J Surg Res 1999;82:180-87.
- Jagroop IA, Burnstock G, Mikhailidis DP. Both the ADP receptors P2Y1 and P2Y12, play a role in controlling shape change in human platelets. Platelets 2003;14:15-20.
- Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res 2006;99:1293-304.
- Wu CC, Tsai FM, Chen ML, Wu S, Lee MC, Tsai TC, et al. Antipsychotic Drugs Inhibit Platelet Aggregation via P2Y 1 and P2Y 12 Receptors. Biomed Res Int 2016;2016:2532371.
- Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood 2005;105:3552-60.
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 2004;113:340-5.
- Roger S, Pawlowski M, Habib A, Jandrot-Perrus M, Rosa JP, Bryckaert M. Costimulation of the Gi-coupled ADP receptor and the Gq-coupled TXA2 receptor is required for ERK2 activation in collagen-induced platelet aggregation. FEBS Lett 2004;556:227-35.
- Shankar H, Garcia A, Prabhakar J, Kim S, Kunapuli SP. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J Thromb Haemost 2006;4:638-47.
- Paul BZ, Jin J, Kunapuli SP. Molecular mechanism of thromboxane A(2)-induced platelet aggregation. Essential role for p2t(ac) and alpha(2a) receptors. J Biol Chem 1999;274:29108-14.
- FitzGerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol 1991;68:11B-15B.
- Lu Y, Li Q, Liu YY, Sun K, Fan JY, Wang SC, et al. Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases. Sci Rep 2015;5:13824.
- Zou ZW, Xu LN, Tian JY. [Antithrombotic and antiplatelet effects of rosmarinic acid, a water-soluble component isolated from radix Salviae miltiorrhizae (danshen)]. Yao Xue Xue Bao 1993;28:241-5.
- Chapado L, Linares-Palomino PJ, Salido S, Altarejos J, Rosado JA, Salido GM. Synthesis and evaluation of the platelet antiaggregant properties of phenolic antioxidants structurally related to rosmarinic acid. Bioorg Chem 2010;38:108-14.
- Huang ZS, Zeng CL, Zhu LJ, Jiang L, Li N, Hu H. Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. J Thromb Haemost 2010;8:1383-93.
- Wu YP, Zhao XM, Pan SD, Guo DA, Wei R, Han JJ, et al. Salvianolic acid B inhibits platelet adhesion under conditions of flow by a mechanism involving the collagen receptor alpha2beta1, Thromb Res 2008;123:298-305.
- Schror K. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost 1997;23:349-56.
- Fukawa K, Saitoh K, Irino O, Ohkubo K, Hashimoto S. Inhibitory mechanism of dipyridamole on platelet aggregation ex vivo. Thromb Res 1982;27:333-40.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003;3:113-22.
- Stangl PA, Lewis S. Review of Currently Available GP IIb/IIIa Inhibitors and Their Role in Peripheral Vascular Interventions. Semin Intervent Radiol 2010;27:412-21.
- Paikin JS, Eikelboom JW. Cardiology patient page: Aspirin. Circulation 2012;125:e439-42.