- *Corresponding Author:
- S. Varun
Delhi Institute of Pharmaceutical Sciences and Research, Pushp Vihar, New Delhi-110 017, India
E-mail: varunsinghbiochem@yahoo.co.in
| Date of Submission | 30 January 2017 |
| Date of Revision | 03 September 2017 |
| Date of Acceptance | 15 April 2018 |
| Indian J Pharm Sci 2018;80(3):565-570 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Moringa oleifera Lam. extract appeared to boost the pregnancy while some literature reported abortive activity causing a dilemma for the users. Present study was conducted to rule out the fertility enhancement effect of the extract. Ethanol extract was evaluated at dose levels of 100, 250, 500 mg/kg on fertility, implantation, decidualization and local cytokine signaling during decidualization in female Wistar rats with artificially induced decidualization. Moringa oleifera at dose levels of 250 and 500 mg/kg led to defective implantation with smaller and dull in color implants compared to the control group which could be due to defective decidualization. Further, artificial decidualization studies revealed that the dose-dependent reduction in weight gain, estradiol levels and progesterone levels, which led to reduced expression of various local cytokines, cyclooxygenase-2, leukemia inhibitory factor, vascular endothelial growth factor and interleukin 11 as became evident by immunohistochemistry and reverse transcription polymerase chain reaction studies. The present study revealed that Moringa oleifera ethanol extract at dose level 250 and 500 mg/kg produced antifertility activity by inhibiting the implantation in female rats, which required further evaluation for potential contraceptive action.
Keywords
Antifertility, Moringa oleifera, implantation, organogenesis, decidualization
Increase in population leads to tremendous pressure on the natural resources and extra burden for policy makers for planning potable drinking water, food, accommodation, education, and infrastructure [1]. This scenario becomes worst in case of developing countries as those are still trying to cope-up with their current situation. Increasing the natural resources is not possible while controlling population growth is possible. Although there are a variety of contraceptive, interceptive drugs and devices available in the market, adverse drug reactions and safety for their long term use is a concern. Moringa oleifera Lam. is the native of South-East Asia and grows well in tropical and subtropical province. Its leaves, bark, roots, seeds and other parts are used for dietary and therapeutic purposes [2].
Therapeutic uses reported for Moringa included antibacterial, antiparasitic, antiprotozoan, antihypertensive, antispasmodic, antiulcer, antiinflammatory, hypocholesterolemic and hypoglycaemic [3]. All these claims appear to be due to primarily scientific research and there is a need to thoroughly investigate these claims more through evidence-based research so as to make therapy with this plant safe and effective. Some researchers have reported M. oleifera to be abortifacient [3] whereas, some others reported that it is beneficial for pregnancy [4], which created a dilemma in the minds of the end users. To obtain clarity about the actual effect possessed by M. oleifera leaf, an attempt was made in this investigation to study it’s antifertility activity in Wistar female rats. Previous studies from our laboratory revealed that ethanol extract was more potent than methanol and hydroalcoholic extract.
Fresh leaves of M. oleifera were collected from the Botanical Garden, City Forest, Saket, New Delhi. The leaves were dried under the shade at a temperature of 30±2° and were authenticated at the Department of Botany, National Institute of Science Communication and Information Resources (NISCAIR), reference no NISCAIR/RHMDC/Consult/2013-14 1536/134, New Delhi, India. Leaves were powdered in an electric grinder and thereafter 2.0 kg of ground powder was extracted with 2000 ml ethyl alcohol for 48 h. The filtrate (2.0 l) was concentrated under reduced pressure at 37° to yield 18.3 g of extract (18.30 % w/w) and stored in a sealed dark air tight plastic container at 4-8°, until use. The crude residue suspended in 0.5 % gum acacia served as the dose formulation during experimentation.
Female Wistar rats that were 25-26 d old, were used for artificial decidualization study while adult female rats of proven fertility and weighing around 220- 280 g for the antifertility study. Rats were procured from the institutional animal house. Animals were housed at a temperature of 25±2° with a 12-h light/ dark cycle under standard hygienic conditions and had free access to fresh tap water and feed. The study was approved and conducted as per Institutional Animal Ethics Committee (IAEC/DIPSAR/2010-I/02) of DIPSAR, New Delhi.
Antifertility activity of ethanol extract of M. oleifera leaf was assessed using the procedure as described previously [5]. Female Wistar rats of proven fertility in their oestrus stage were mated with proven fertile male Wistar rats in the ratio of one male with three female rats. On the next day, vaginal smear (early morning) showing sperms was considered as first day of the pregnancy and that female rats were separated from male rats and was subsequently randomly divided into four groups (six animals in each group). Group I, II and III received 100, 250 and 500 mg/kg ethanol extract of the leaf and the control group received 0.5 % gum acacia for 1-7 d post coitus. Animals were laparotomized on 10th d, number of implants occur in both the uterine tube as and numbers of corpora luteum (CL) on respective ovaries were counted. Then female rats were allowed to deliver litters at the end of the same gestation period (21-23 d) and the number of litters delivered were recorded, Pre-implantation and postimplantation loss was calculated using the following formulae [5]. Pre-implantation loss = number of CL on 10th d–number of implants on 10th d; post-implantation loss = number of implants on 10th d–number of litters delivered; percent pre-implantation loss = (number of CL–number of implants)×100/CL; percent postimplantation loss = (number of implants–number of litters delivered)×100/number of implants.
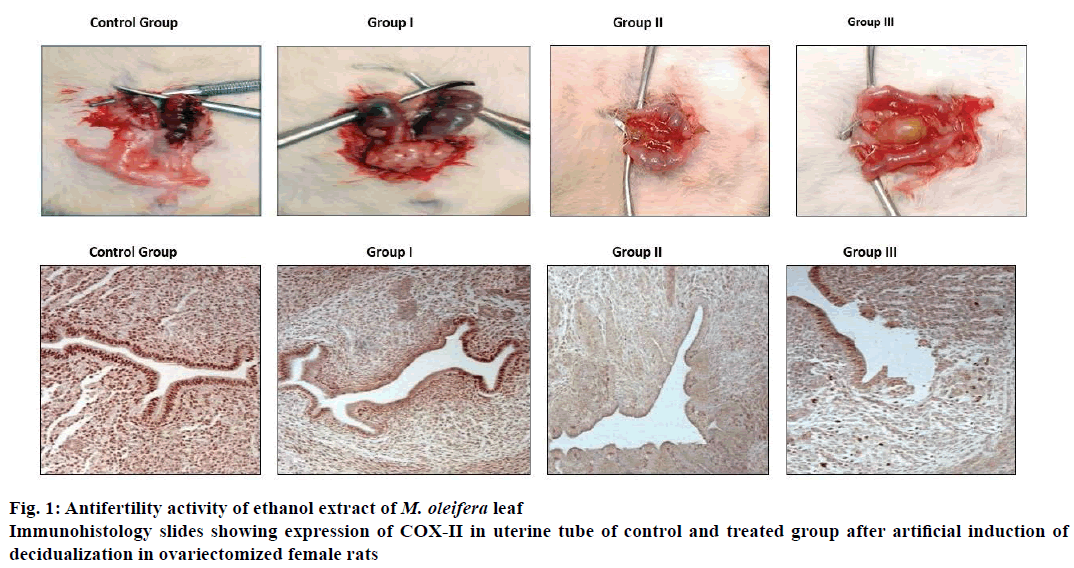
Artificial decidualization was induced by intraluminal injections, female rats (6 animals in each group) weighing approximately 180 g were ovariectomized. One week after ovariectomy, animals were started on a hormone regimen wherein three daily subcutaneous injections of 17β-oestradiol (100 ng/d/rat) for 3 d (days 1-3), no treatment on days 4 and 5, progesterone (1 mg/d/rat) plus 17β-oestradiol (10 ng/d/rat) on days 6-8. Group I, II and III received 100, 250 and 500 mg/kg ethanol extract of M. oleifera leaf from day 5 to 9, control group female rats received 0.5 % gum acacia for 5-9 d. The induction of deciduoma is initiated on day 8 by infusing 40 μl sesame seed oil inside one uterine lumen. Rats were sacrificed by cervical dislocation on day 9 and whole uteri were cleaned of fat and weighed. A part of decidualized uterine tube from each animal of all groups was fixed in formalin saline solution, embedded in paraffin wax, sectioned and used for immunohistochemistry study [6] and rest of decidualized uterine tube part was preserved in liquid nitrogen for subsequent RT-PCR studies and hormone and protein assay.
Uterine tissue sections of 5.0 μm thick were deparaffinised, rehydrated and stained for immunohistochemistry as described previously [6]. Tissue sections were heated in 10 mM citrate buffer with 0.1 % (v/v) Triton X-100 (Sigma-Aldrich), then washed with phosphate buffer solution and incubated with 0.3 % methylated hydrogen peroxide to quench endogenous peroxide activity and subsequently with secondary washing buffer and incubated with blocking serum at 37° for 1 h. Thereafter, the blocking was done with primary antibody COX-II (Abcam) and the tissue sections were washed in buffer and incubated with subsequent antibody, washed again with buffer and incubated with avidin-biotin complex and horseradish peroxides for 30 min and counterstained with haematoxylin.
One part of frozen uterine tissue was weighed and homogenized in phosphate buffer saline (10 %) as described previously [6]. The homogenate obtained was processed as follows: 500 μl were centrifuged at 2000×g for 10 min at 4° and subsequently oestradiol and progesterone determination was done by ELISA kits and protein concentration by Bradford method. RNA was isolated from the second half of the uterine tissue using a method described previously with minor modification [6] with TRIzol reagent for expression of IL-11 and leukaemia inhibitory factor (LIF). RNA was re-suspended in 75 % diethylpyrocarbonate and quantified (A260 nm). One microgram of total RNA was reverse-transcribed to cDNA in a final volume of 20 μl, according to the manufacturer's instruction.
For real-time quantification of selected mRNA transcript levels of LIF, VEGF and IL-11 in uterine tissue at 48 h of decidualization, PCR was carried out as previously described [6]. The relative changes in LIF, VEGF and IL-11 with respect to β-actin expressions were examined using the ΔΔCt method, ΔCt = Cttarget– Ct βactin.
All results were analysed statistically student-t test and one-way analysis of variance (ANOVA), statistically significant effects were evaluated each group (n=6) with Newman-Keuls tests. Differences were considered significantly at p<0.01. Results were expressed as means±SD using GraphPad prism 5 software.
Ethanol extract of M. oleifera leaf at a dose level of 100, 250 and 500 mg/kg post coitus from day 1 to 7 showed marked antifertility activity compared to control group. Antifertility activities in the treated groups were dose-dependent and major cause of inhibition in fertility was due to pre-implantation loss. M. oleifera at a dose of 100 mg/kg did not show any significant difference in antifertility post-implantation compared to control but, at the dose of 250 mg/kg showed marked antifertility activity compared to control group whereas, and at the dose of 500 mg/kg showed 100 % antifertility activity as shown in Table 1. Ethanol extract of M. oleifera leaf at doses of 250 and 500 mg/kg resulted in defective implantation as shown in Figure 1A. The implants were smaller in size and dull in colour compared to those of the control group.
| Drug treatment | Mean number of corpus luteum (n=6) | Mean number of implants (n=6) | Mean number of litters (n=6) | % pre-implantation loss (n=6) | % post-implantation loss (n=6) | % antifertility activity (n=6) |
|---|---|---|---|---|---|---|
| Control group | 16.83±1.16 | 13.16±0.75 | 10.83±0.75 | 18.18±7.73 | 17.70±3.64 | 32.76±5.83 |
| Group I | 16.66±1.03 ns | 10.50±0.54a** | 8.6±0.51a** | 36.78±5.44a*** | 17.42±3.72a,ns | 47.84±4.42a** |
| Group II | 16.50±1.04 ns | 9.33±0.81a**,b,ns | 6.30±0.51a***,b** | 42.20±6.29a***, b* | 31.94±4.99ab*** | 61.42±4.74a***b** |
| Group III | 16.50±1.04 ns | 4.33±1.36abc*** | Nilabc*** | 73.53±8.80abc*** | 100abc*** | 100abc*** |
*Significantly different from n=6 in each group at p<0.01, results were expressed in mean±SD multiple comparison test where, a- between control vs. treated group I, II and III, b- between treated group I vs. group II and III, c- between treated group II vs. group III , ns- non-significant
Table 1: Antifertility activity of ethanol extract of Moringa oleifera leaf
Ethanol extract of M. oleifera leaf at doses of 100, 250 and 500 mg/kg body weight showed dose-dependent reduction in induced deciduloma weight gain of uterine tissue compared to control group as shown in Table 2. This is further supported by immunohistochemical staining for COX II. Slides demonstrated reduction in COX-II expression and inhibition in proliferation of deciduloma cells as shown in Figure 1B, uterine tissue morphology showed widening of uterine lumen as compared to control group evident proliferating deciduloma cells occupied more space and leads to narrowing of uterine lumen. Ethanolic leaf extract of M. oleifera showed reduction in localized uterine tissue oestradiol and progesterone levels compared to control group. Reduction in uterine tissues oestradiol levels at dose level of 100, 250 and 500 mg/kg are 82.93, 75.98 and 58.58 %. Similarly, progesterone levels were 84.07, 77.56 and 62.20 % compare to control group as shown in Table 2.
| Parameters | Control group | Group I | Group II | Group II |
|---|---|---|---|---|
| Folds Increased in uterine weight | 14.41±1.769 | 10.22±1.107a*** | 8.13±0.841a***b* | 6.53±0.680abc*** |
| Progesterone (ng/mg protein) |
269.97±7.122 | 226.97±9.720a*** | 209.39±5.492a***b** | 167.94±6.267abc*** |
| Oestradiol (ng/mg protein) | 178.06±8.763 | 147.67±5.626a*** | 135.30±2.344a***b* | 104.32±2.249abc*** |
*Significantly different from n=6 in each group at p<0.01, results were expressed in mean±SD multiple comparison test where, a- between control vs. treated group I, II and III, b- between treated group I vs. group II and III, c- between treated group II vs. group III
Table 2: Uterine weight gain, progesterone and estradiol levels in control and ethanol extract of Moringa oleifera leaf-treated group after artificial induction of decidualization in ovariectomized female rats
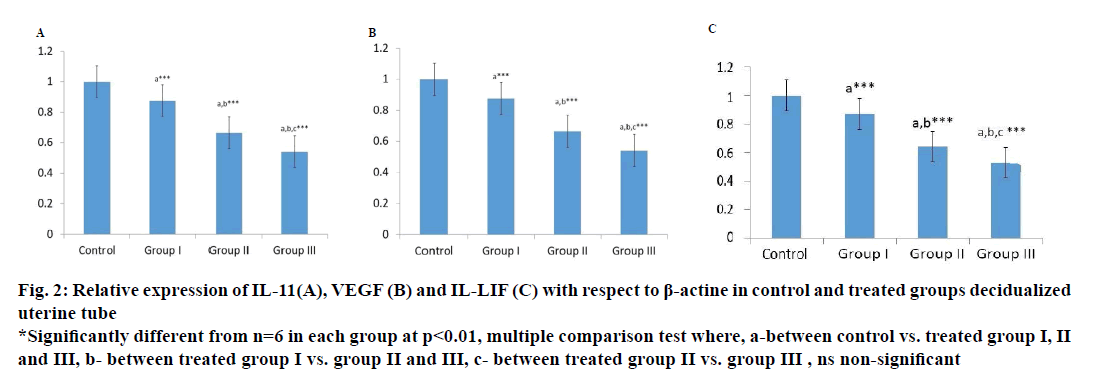
Localized expression of IL-11, VEGF and LIF gene in uterine tissues after artificial induction of decidualization of is marked down regulated in the ethanolic leaf extract of M. oleifera-treated group compare to control group as shown in Figure 2, IL-11 levels of expression in uterine tissue at dose levels 100, 250 and 500 mg/kg body weight were 87.0, 66.48 and 54.15 %. Similarly, VEGF was 88.8, 70.8 and 56.80 % and LIF was 87.00, 64.33 and 52.83 % compared to control group indicating a marked interruption in cytokines signalling pathway.
Figure 2: Relative expression of IL-11(A), VEGF (B) and IL-LIF (C) with respect to β-actine in control and treated groups decidualized
uterine tube
*Significantly different from n=6 in each group at p<0.01, multiple comparison test where, a-between control vs. treated group I, II
and III, b- between treated group I vs. group II and III, c- between treated group II vs. group III , ns non-significant
Ethanol extract of M. oleifera leaf showed antifertility activity at dose levels of 100, 250 mg/kg and complete antifertility activity at the dose of 500 mg/kg on oral administration from post coital days 1-7. Antiimplantation loss at each dose was more than the post-implantation loss, which led to the conclusion that the extract of M. oleifera interfered with the implantation process in Wistar rats. Further studies were performed to examine the effect of ethanol extract of M. oleifera leaf on decidualization initiated by progesterone and 17β-oestradiol supplementation and sesame seed oilinduced artificial decidualization. Results obtained indicated that there was a reduction in proliferation of deciduloma cells in the treated group compared to the control group as wider lumen space and reduction in uterine biomass were observed. Implantation is the process in which blastocyst comes into intimate physical and physiological contact with the uterine endometrium. Implantation event [7] may be divided into three stages, apposition, adhesion and penetration. During the penetration stage, invasion of the luminal epithelium by the stromal cell via proliferation and differentiation into decidual cells, a process called decidualization. Results of this investigation, as well as the COX-II immunostaining findings further supported that there was marked reduction in expression of COX-II in luminal epithelium and endometrial stoma cells COX-II. COX is the ratelimiting enzyme in the biosynthesis of prostaglandins, which are important mediators causing increased endometrial vascular permeability during implantation and decidualization [8]. COX-II expression increased during implantation at the site of luminal epithelium and underlying stromal cells. Further, the analysis of uterine tissue progesterone and estrogen levels showed a dose-dependent reduction in the treated group compared to control group as shown in Table 2, which indicated the inhibition in localized steroidal hormone. This local oestrogen is required for the induction of progesterone receptors and progesterone is an absolute requirement for sustained decidualization, absence of which resulted in embryo resorption [9]. These results are fully permissible with the previous studies reporting that M. oleifera extracts demonstrated antioestrogenic and antiprogestogenic effects [10,11]. Further, analysis of uterine tissue expression of IL-11, LIF, and VEGF showed marked reduction compared to control uterine tissue levels. These cytokines and their receptors in the uterus were involved in various aspects of implantation. VEGF is responsible for angiogenesis required for blood vessel development from pre-existing vessels, which is essential in the uterus for successful implantation, decidualization, and placentation. IL-11 is crucial to decidualization but not for attachment and similarly, LIF is crucial for activation of blastocyst and implantation. IL-11 and LIF belong to IL-6 super family and both have a common glycoprotein gp130 signal transduction partner and uterine hormones were considered to regulate the expression of LIF and VEGF and any aberrant in LIF expression leads to reduced COX-II expression. Current investigation indicated that M. oleifera extract at 500 mg/kg dose induced antifertility activity by interfering with implantation and decidualization process perhaps through its antioestrogenic and antiprogestogenic effect, which led to interruption of the cyclic cytokines cascade of COX-II, VEGF, LIF and IL-11 but did not throw any light on the key constituent of the ethanol extract of M. oleifera leaf which could have been responsible for the antifertility activity and also its usefulness as an antifertility agent due to the fact that no standard drug was included for comparison. Hence further studies with M. oleifera are necessary to identify the active constituent responsible for antifertility activity in comparison with a standard drug.
The present study revealed that ethanol extract of M. oleifera at a dose of 500 mg/kg produced antifertility by inhibiting the implantation and also raised a query about its usefulness during the pregnancy. This investigation further suggested that a comprehensive study is required to identify key active constituent responsible for the antiimplantation activity.
Acknowledgments
Authors are grateful to the Department of Training and Technical Education, Government of NCT Delhi for the financial support.
Financial support and sponsorship
Nil.
References
- https://www.geni.org/globalenergy/research/population-picture/population-picture.pdf, October 2011.
- Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res 2015;29:796-804.
- Ekhator CN, Osifo UC. Abortifacient efficacy of Moringa oleifera Leave: An Experimental study on adult female Wistar Rats. Am J Biol Life Sci 2015;3:269-72.
- Sindhu S, Mangala S, Sherry B. Efficacy of Moringa oleifera in treating iron deficiency anemia in women of reproductive age group. Int J Phytother Res 2013;4:15-20.
- Agrawal SS, Kumar A, Gullaiya S, Dubey V, Singh. V, Tiwari P. Antifertility activities of methanolic bark extract of Aegle marmelos (L.) in male Wistar rats. Daru 2013;20(1):94.
- Agrawal SS, Kumar A, Gullaiya S, Dubey V, Singh V, Nagar A. Contraceptive activity of 4-(4-hydroxy-3-methyl-hex-5-enyl)-chroman-2,7-diol via inhibiting ovulation in gonadotropin-primed immature rat model. Biomed Aging Pathol 2014;43-7.
- Walter B, Emile B. Medical physiology: A cellular and molecular approach. 2nd ed. Philadelphia, PA: Saunders/Elsevier; 2009.
- Haibin W, Sudhansu KD. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006;185-99.
- Paria BC, Jian T, Dennis BL, Dev SK, Das SK. Uterine decidua response occurs in estrogen receptor-α deficient mice. Endocrinology 1999;140:2704-10.
- Shukla S, Mathur R, Prakash OA. Antifertility profile of the aqueous extract of Moringa oleifera roots. J Ethanopharmacol 1988;22:51-62.
- Varsha Z, Dinesh D. Antifertility effect of alcoholic extract of Moringa oleifera stem bark on estrous cycle and estrogenic activity of female Albino rat. Adv Drug Deliv Rev 2015;223-35.